Chapter 1 Introduction: Matter and Measurement
advertisement
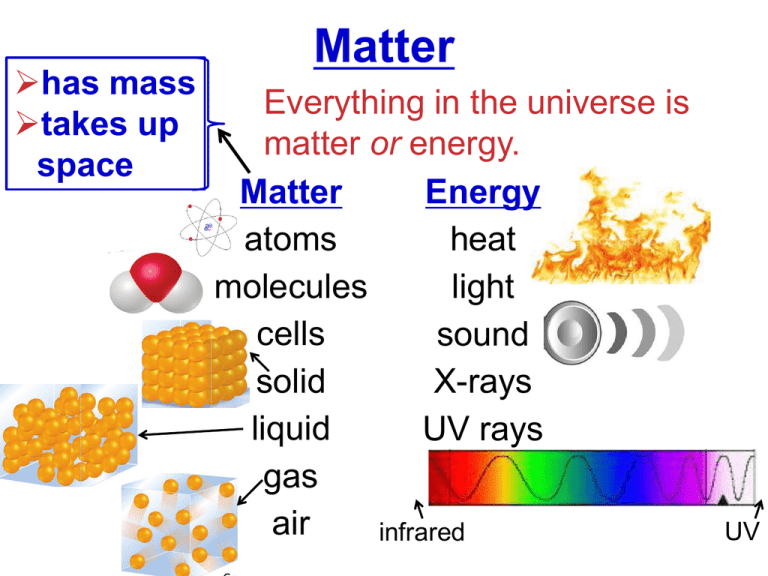
has mass takes up space Matter Everything in the universe is matter or energy. Matter Energy atoms heat molecules light cells sound solid X-rays liquid UV rays gas air infrared UV States of Matter SOLID LIQUID GAS low KE, vibrates together higher KE, flows together highest KE, expands apart Properties of Matter • Physical Properties: observed without changing into new substance. • temp., state, mp, bp, color, mass, volume, etc. • Chemical Properties: only be observed when changed into new substance. • flammability, corrosiveness, reactivity, etc. Properties of Matter Copper (Cu) Physical ??? Chemical ??? Properties of Metals & Nonmetals Metals Nonmetals (sodium, iron, copper, zinc) (sulfur, oxygen, neon, carbon) Luster (shiny) Brittle Malleable (sheets) Physical _________ Ductile (wires) Properties: Good Conductors Poor Conductors (heat/electricity) Metalloids Quick Quiz! 1. Which of the following is NOT a physical property of matter? A. melting point B. color C. reactivity D. hardness Quick Quiz. 2. Which properties can be observed only by changing the composition of a substance? A. all properties of a substance B. intensive properties C. chemical properties D. physical properties Quick Quiz. 3. Match the states of matter with the following descriptions: (1) particles expand apart (2) vibrates in place (3) particles move around but stay in contact A. (1) liquid, (2) solid, and (3) gas B. (1) gas, (2) solid, and (3) liquid C. (1) gas, (2) liquid, and (3) solid