Chemistry Matter and Change
advertisement

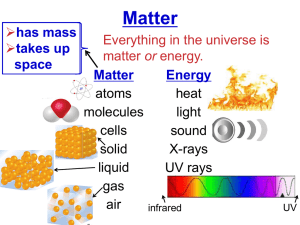
Chemistry Matter and Change Chemistry is… …the study of the composition, structure, and properties of matter and the changes it undergoes C2H5OH + 3 O2 2 CO2 + 3 H2O + Energy Reactants Products Matter Anything that has mass and occupies space Mass A measure of the amount of matter Atom The smallest unit of an element that maintains the properties of that element Element A pure substance made of only one kind of atom Compound A substance that is made from the atoms of two or more elements that are chemically bonded. Sucrose – C12H22O11 Sucrose is also known as table sugar, and is used to make Gummy Bears! Properties of Matter Extensive properties depend on the amount of matter that is present. Volume Mass Energy Content (think Calories!) Intensive properties do not depend on the amount of matter present. Melting point Boiling point Density Physical Change A change in a substance that does not involve a change in the identity of the substance. Example: Phase Changes Phase Differences Solid – definite volume and shape; particles packed in fixed positions. Liquid – definite volume but indefinite shape; particles close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun. Three Phases Copper Phases - Solid Copper Phases - Liquid Copper Phases – Vapor (gas) Chemical Change A change in which one or more substances are converted into different substances. Heat and light are often evidence of a chemical change. Classification of Matter Separation of a Mixture The constituents of the mixture retain their identity and may be separated by physical means. Separation of a Mixture The components of dyes such as ink may be separated by paper chromatography. Separation of a Mixture Distillation Separation of a Compound The Electrolysis of water Compounds must be separated by chemical means. With the application of electricity, water can be separated into its elements Reactant Water H2O Products Hydrogen + Oxygen H2 + O2 Group or Family Period The Periodic Table Group or family Period Properties of Metals Metals are good conductors of heat and electricity Metals are malleable Metals are ductile Metals have high tensile strength Metals have luster Examples of Metals Potassium, K reacts with water and must be stored in kerosene Copper, Cu, is a relatively soft metal, and a very good electrical conductor. Zinc, Zn, is more stable than potassium Mercury, Hg, is the only metal that exists as a liquid at room temperature Properties of Nonmetals Carbon, the graphite in “pencil lead” is a great example of a nonmetallic element. Nonmetals are poor conductors of heat and electricity Nonmetals tend to be brittle Many nonmetals are gases at room temperature Examples of Nonmetals Sulfur, S, was once known as “brimstone” Graphite is not the only pure form of carbon, C. Diamond is also carbon; the color comes from impurities caught within the crystal structure Microspheres of phosphorus, P, a reactive nonmetal Properties of Metalloids Metalloids straddle the border between metals and nonmetals on the periodic table. They have properties of both metals and nonmetals. Metalloids are more brittle than metals, less brittle than most nonmetallic solids Metalloids are semiconductors of electricity Some metalloids possess metallic luster Silicon, Si – A Metalloid Silicon has metallic luster Silicon is brittle like a nonmetal Silicon is a semiconductor of electricity Other metalloids include: Boron, B Germanium, Ge Arsenic, As Antimony, Sb Tellurium, Te