Metals
advertisement

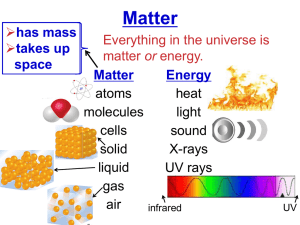
The Periodic Table Metals, Metalloids, and Nonmetals Metals * Group 1 are the Most REACTIVE metals * Transition Metals Group 3 - Group 12 Physical Properties Chemical Properties 1. Conductors (Heat and Electricity) 2. Have Luster (Shiny) 3. Malleable (rolled or flattened) 4. Ductile (pulled in a wire) 5. Solids at Room Temp *Except Mercury, symbol Hg 1. Metals Corrode Corrosion: the deterioration of metals due to a chemical reaction with the environment Ex. Iron & Oxygen = Rust Nonmetals • Group 17 most Reactive Nonmetals (the Halogens) • Group 18 least Reactive (the Nobel Gases) • Life on Earth Depends on Nonmetals (H,C,N,O) Physical Properties Chemical Properties 1. Poor Conductors 2. Dull (not shiny) 3. Brittle (Break easily) 4. Mostly Gases, a few Solids *Except Bromine is a Liquid 1. Gain Electrons easily due to location in the higher groups Diatomic Molecule: Made up of two atoms Ex. Oxygen = O2 Hydrogen = H2 Metalloids They are the Staircase Elements • Contain some properties of both metals and nonmetals • All Solids at room temperature Metalloids are Semiconductors. They can conduct electric currents under some conditions, but not under others.