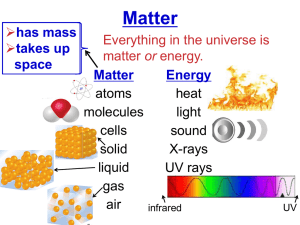
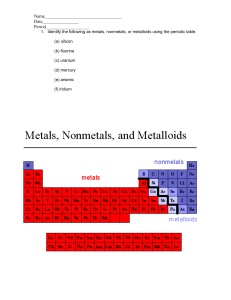
Name: ________________________________ Period: ______________ LABORATORY ACTIVITY: METAL OR NONMETAL? Introduction: Elements can be grouped or classified in several ways according to similarities and differences in their properties. Two major classes are metals and nonmetals. Everyday experience has given you some knowledge of the properties of metals. In this laboratory activity you will have a chance to explore further the properties of metals and nonmetals. Several elements are not clearly metals or nonmetals; they have properties that are intermediate. These elements are called metalloids. In some properties metalloids are like metals and in others they are like nonmetals. Every element can be classified as a metal, a nonmetal, or a metalloid. What properties of matter are involved in this classification? This activity will help you find out. Purpose: In this activity you will investigate properties of several elements in order to classify them as metals, nonmetals or metalloids. You will examine each for its physical properties of color, luster, and form (for example, is it crystalline, like table salt?). By attempting to crush each sample with a hammer, you will decide whether each element is malleable or brittle. You will also test for the physical property of electrical conductivity. Next, you will observe differences among these elements’ chemical properties. You will find out whether each element reacts with hydrochloric acid, HCl (aq), and with a copper (II) chloride (CuCl2) solution. Procedure: Properties 1. Appearance: Observe and record the appearance of each element. Include physical properties such as color, luster, and also the form. 2. Conductivity: Use a conductivity apparatus to test each sample. Caution: Avoid touching the electrodes with your hands: you can get an electric shock. Touch both electrodes to the element sample. If the bulb lights, the sample has allowed electricity to flow through it. Such a material is called a conductor. If the bulb fails to light, the material is a nonconductor. 3. Crushing: A material is malleable if it flattens without shattering when struck. A sample is brittle if it shatters into pieces. Gently rap each element sample with a hammer. Decide whether the samples are malleable or brittle. 4. Reactivity with HCl: Test each sample for reactivity with acid as described below. The formation of gas bubbles indicates that a chemical reaction has taken place. a. Place a well plate over a piece of paper. Label ten of the wells a to j. b. Place a sample of each element in its appropriately labeled well. The sample should be a 1 cm length of wire or ribbon, or 0.5-1.0 g of solid. c. Add 20 drops of hydrochloric acid (HCl) to each tube. d. Observe and record each result. e. Disposal: Decant the liquid from the tubes into the liquid waste container provided by your teacher and dispose of the solids in the solid waste disposal. Rinse out the tubes with water for the next step. 5. Reactivity with CuCl2: Test each element sample for reactivity with copper (II) chloride (CuCl2) solution as described below. Changes in the appearance of any element sample indicate a chemical reaction has taken place. a. Repeat steps 4a and 4b. b. Add approximately 20 drops of CuCl2 solution to each well. c. Observe the well plate for five minutes – changes may be slow. d. Disposal: Decant the liquid from the tubes into the liquid waste container provided by your teacher and dispose of the solids in the solid waste disposal. Rinse out the tubes with water for the next step. 6. Wash your hands thoroughly before leaving the laboratory. Lab Write-Up: Type your observations into a data table on Excel to turn in. Data tables should have a column for each property observed (conductivity, color, reactivity, etc.). Answer the following questions to turn in with your data table. Questions: 1. Using your data, sort the provided elements into groups of metals, nonmetals and metalloids, based on their similarities and differences in their physical and chemical properties. 2. Are there any inconsistencies with the groups you made (is any of your data incorrect based on how they are organized on the periodic table)? Do any elements seem to have properties of both groups? Which? Explain. 3. Look at the location on the periodic table of each of the elements tested in this lab. How do the properties of these elements compare to their general position on the periodic table? Make generalizations about the position of the metals, nonmetals, and metalloids on the periodic table. 4. Predict the physical and chemical properties of the following elements which were not tested in this lab: selenium, calcium, cobalt Data Table: Element Al S Si Sn Pb Mg Cu Ni Zn C Observations