Electrochemistry - APchem-MCC
advertisement

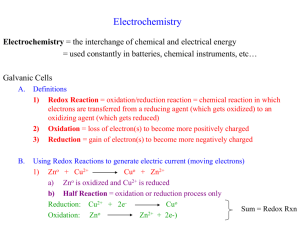
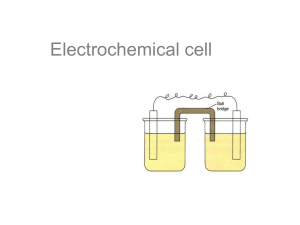
Electrochemistry Chapter 20 Introduction • Electrochemistry involves looking at how spontaneous redox reactions can be used to produce electricity and how electricity can cause nonspontaneous reactions to occur. • Ex. Batteries use spontaneous redox reactions and convert chemical energy into electrical energy • Review of redox reactions: • A species is oxidized when it loses electrons. – Zinc loses two electrons, forming the Zn2+ ion. • A species is reduced when it gains electrons. – H+ gains an electron, forming H2. 20.2 – Balancing Redox Equations • Half-Reactions = either the oxidation or the reduction process shown alone with the # of electrons lost or gained. • Ex. Cu(s) → Cu2+(aq) + 2e- (oxidation) • Ex. Ag+(aq) + e- → Ag(s) (reduction) • The # of electrons lost must equal the # gained, so the second half-reaction becomes -- 2Ag+(aq) + 2e- → 2Ag(s) • When you add them together, you get an oxidation-reduction reaction: • Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s) • For acidic solutions, follow the steps on the bottom of pg 860 and the top of page 861 • Sample exercise 20.2 • Balancing in Basic Solution: • A reaction that occurs in basic solution can be balanced as if it occurred in acid. • Once the equation is balanced, add OH– to each side to “neutralize” the H+ in the equation and create water in its place. • If this produces water on both sides, subtract water from each side so it appears on only one side of the equation. • Sample exercise 20.3 20.3 – Voltaic Cells • In spontaneous redox reactions, electrons are transferred and energy is released. • That energy can do work if the electrons flow through an external device. • This is a voltaic cell. • Components of a Voltaic Cell: electrode = a strip of metal used in an electrochemical experiment. It can be made of a metal that’s in the reaction, or another conducting material such as platinum. a) anode = the electrode at which oxidation occurs b) cathode = the electrode at which reduction occurs half-cell = one of the compartments of a voltaic cell Salt bridge = a U-shaped tube containing an electrolyte solution that neutralizes any charge buildup in the half-cells. Electrons flow through the wire from the anode to the cathode, producing an electrical current. • See sample exercise 20.4 • Important other points about a voltaic cell: If the electrodes are made of materials that participate in the reaction (i.e. a zinc electrode in a solution of ZnSO4), than the anode will lose mass as the neutral atoms of the electrode oxidize into ions, and the cathode will gain mass as its ions deposit as neutral atoms. If the electrodes are made of a conducting material that does not participate in the reaction, their masses don’t change. The cations in the salt bridge will migrate to the cathode to neutralize the negative charge that will build up at the + ions become neutral, leaving the −ions (spectators) in excess. The anions in the salt bridge will migrate to the anode to neutralize the excess + charge from the ions that are forming. 20.4 – Cell Potentials Under Standard Conditions (E0cell) • In a Voltaic cell, electrons flow spontaneously from the anode to the cathode because of a difference in potential energy (they flow from high to low potential energy). • The difference in potential energy between the 2 electrodes (called the cell potential, or Ecell) is measured in volts. Note: your book also calls it emf • One volt is one joule per coulomb (1 V = 1 J/C). An electron has a charge of 1.60 x 10-19 C. • E°cell is the standard cell potential [occurs at standard conditions – 1M (or 1atm for gaseous) reactants and products]. • Standard reduction potential (E°red): • It’s a measurement of the tendency for a reduction process to occur at an electrode. The value is determined by comparison to a standard hydrogen electrode (H+→H2), which is given a value of 0. – – Substances that are easily reduced have a large + value for their E°red. Substances that are easily oxidized have a large – value for their E°red. • To calculate E°cell: 1) write the reduction half-equation and look up its E°red 2) write the oxidation half-equation and write the opposite sign of its E°red (which is a E°oxid) 3) combine the half-equations and add the E°s. Note: E° values are unaffected by coefficients. • Voltaic cells will have a + E°cell • See sample exercises 20.5 – 20.7 • The larger the + value for the E°red, the more easily the substance is reduced • The more negative the value is for E°red, the more likely the substance will be oxidized. • **metals at the top of the activity series have the most negative E°red 20.5 – Free Energy and Redox Reactions • • • • • • • Relationship of Ecell to spontaneity: If Ecell is +, the rxn is spontaneous in the forward direction If Ecell is -, the rxn is spontaneous in the reverse direction If Ecell is 0, the rxn is at equilibrium See sample exercise 20.9 Relationship of Ecell to free energy: Go = -nFEocell , where n = the # of moles of electrons transferred according to the balanced equation, and F = the electric charge per mole of e-, called the Faraday constant (96,485 C/mole e- = 96485J/V-mol). The units for Go are J/mol • Relationship between ΔG, Eocell , and K: • ΔG° = –nFE° = –RT ln K • See sample exercise 20.10 20.6 – Cell Potentials Under Nonstandard Conditions • Effect of Concentrations on Cell Voltage: • Most cell measurements involve nonstandard conditions (i.e. not 1M solute concentrations and/or not 1 atm) • Using nonstandard conditions will give you an Ecell that is different from the E°cell. Also, the Ecell changes as the reaction progresses. • Ex. Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) • Think of it as an LeChatelier’s problem • As the reaction proceeds, the conc. of products ↑, and the conc. of reactants ↓, so the reaction shifts to the left, and Ecell decreases. Ecell reaches 0 at equilibrium (when the cell is “dead”) • In general, increasing the concentration of the reactants or decreasing the concentration of the products drives the reaction towards the products, increasing Ecell. • Conversely, decreasing the concentration of the reactants or increasing the concentration of the products results in a smaller Ecell. • Note: changing the size of the electrodes has no effect on the Ecell since the concentration of solids doesn’t change (as long as some electrode remains in contact with the solution). 20.9 -- Electrolysis • Electrolytic Cell: Electricity from an external source causes a nonspontaneous reaction to occur (electrolysis). The external source of electricity acts like an “electron pump” and pulls electrons away from the anode, where the oxidation occurs, and pushes them into the cathode, where the reduction occurs. • Example: in this example, Na+ and Cl- are forced to become neutral Na and Cl2. The electrodes are inert. • Electroplating: uses electrolysis to deposit a thin layer of one metal onto another to improve the appearance or resist corrosion. • Quantitative Aspects of Electrolysis: • The quantity of reactant consumed or product formed during electrolysis depends on: • 1. the molar mass of the substance (reactant or product) • 2. the quantity of electric charge used (measured in Coulombs) • 3. the # of electrons transferred • The equation to use is: I = q/t, where I is current (amps), q = the charge (Coulombs), and t = time (seconds) • To calculate the quantitative outcomes of electrolysis, the strategy is as follows: • 1. Solve for q • 2. convert q to moles of e- (1 mole e- = 96,485 C) • 3. change moles of e- to moles of reactant or product • 4. convert moles of reactant or product to the unit needed • see sample exercise 20.14