Unit16withAudio
advertisement

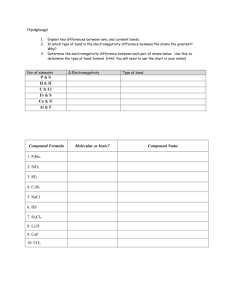
Read Sections 4.7, 4.11 and 4.12 before viewing the slide show. Unit 16 Shapes and Electrical Properties of Molecular Compounds •VSEPR Theory (4.11) •Electronegativity (4.7) •Identification of Compounds as Polar or Nonpolar (4.12) VSEPR Theory (4.11) •The geometry of molecules is important in determining some of their properties •Electrons tend to repel each other because of their negative charges •In considering a Lewis structure for a molecule (or ion) the geometry becomes apparent when one considers that the electrons tend to get as far away from each other as possible •These observations are summarized in the Valence Shell Electron Pair Repulsion theory (VSEPR). Simply stated, electron pairs around a central atom arrange themselves to minimize repulsion. Molecular Shape from VSEPR (4.11) Number of Bonded Atoms Number of Nonbonded Pairs of Electrons Number of Sets of Electrons (sum of bonded atoms and nonbonded pairs) Molecular Shape Examples Picture of Shape 2 3 0 0 2 3 Linear Trigonal Planar HCN, CO2 SO3, BF2Cl O=C=O 2 1 3 Bent SO2 4 0 4 Tetrahedral CH4, CHCl3, CH2Cl2 3 1 4 Pyramidal NH3 2 2 4 Bent H2O Application of the Previous Table (4.11) •Application of the table on the previous slide involves drawing the Lewis structure and determining the number of bonded atoms and nonbonded pairs of electrons •Notice the counting of atoms and nonbonded pairs only involves those around the atom of interest, circled in the table below. Molecule # of Bonded Atoms # of Nonbonded Molecular Shape Pairs around Atom of Interest H▬C≡N| 2 0 Linear .. H▬N ▬H | H 3 1 Pyramidal More Examples (4.11) Molecule # of Bonded Atoms # of Nonbonded Molecular Shape Pairs around Atom of Interest OC O 2 0 Linear H O H 2 2 Bent 4 0 Tetrahedral : Br : | : Br C Br : | : Br : Electronegativity (4.7) •Recall that in ionic compounds electrons are essentially transferred from a metal to a nonmetal •Electrons are not necessarily shared equally between two atoms in a covalent bond •The determining factor as to which atom will attract electrons more in a covalent bond is called the electronegativity •A scale has been set up for electronegativity with fluorine having the highest electronegativity – the strongest ability to draw electrons towards itself in a chemical bond •A section of the electronegativity table is on the next slide Electronegativity and the Periodic Table (4.7) •The chart below gives the electronegativity values of some of the key elements. •A high value (F, for example) means a strong tendency to draw electrons toward that element in a chemical bond •Hydrogen is similar to carbon in terms of electronegativity •The differences in electronegativity between metals and nonmetals are typically large enough to result in a significant transfer of electrons to form ionic compounds 1 2 13 14 15 16 17 18 •The nonmetal-nonmetal bonds H Largest are covalent – they involve 2.1 electron sharing, but not equally Li Be B C N O F 1.0 Na 0.9 K 0.8 1.5 Mg 1.2 Ca 1.0 2.0 Al 1.5 2.5 Si 1.8 3.0 P 2.1 As 2.0 3.5 S 2.5 Se 2.4 4.0 Cl 3.0 Br 2.8 I 2.5 Polar and Nonpolar Covalent Bonds (4.7) •If the difference in electronegativity between the two atoms is less than 0.5, the electrons are about equally shared and the bond is called a nonpolar covalent bond •If the difference in electronegativity between the two atoms is between 0.5 and 2.0, the electrons are drawn toward the more electronegative element and the bond is called a polar covalent bond. Basically the negative and positive charges are separated from each other. •If the difference in electronegativity between the two atoms is greater than 2.0, the bond is considered to be ionic because of a significant transfer of electrons. Bond Diff. in EN Bond Type N-O 3.5 - 3.0 = 0.5 polar covalent Al-Cl 3.0 – 1.5 = 1.5 polar covalent C-H 2.5 - 2.1 = 0.4 nonpolar covalent K-O 3.5 - 0.8 = 2.7 ionic 1 2 13 14 15 16 17 H 2.1 Li 1.0 Na 0.9 K 0.8 Be 1.5 Mg 1.2 Ca 1.0 B 2.0 Al 1.5 C 2.5 Si 1.8 N 3.0 P 2.1 As 2.0 O 3.5 S 2.5 Se 2.4 F 4.0 Cl 3.0 Br 2.8 I 2.5 18 Polar and Nonpolar Covalent Bonds (4.7) •There are a couple of ways of representing in a polar covalent bond which way the electrons are pulled •Use the greek letter δ with a superscript to represent whether it is at the positive or negative end of the bond •Use an arrow such as that points toward the more electronegative atom. The length of the arrow may be used to indicate on a relative basis how big the difference in electronegativity is. The arrow has a size and direction which together can be referred to as the dipole moment. Bond Diff. in EN N-O 3.5 – 3.0 = 0.5 Al-Cl P-F 3.0 – 1.5 = 1.5 4.0 – 2.1 = 1.9 Greek Rep. NO Al Cl P F Arrow Rep. NO Al Cl PF Polar and Nonpolar Molecules (4.12) •A molecule itself may be polar or nonpolar depending upon the geometric arrangement of its bonds. •A polar molecule has separate centers of positive and negative charges, similar to north and south poles of a magnet. •A nonpolar molecule has the centers of positive and negative charges at the same place, similar to a magnet in which the poles have collapsed on top of each other. •The polarity of the bonds and the geometry of the molecule must both be considered to determine whether or not a molecule is polar or nonpolar. CH4 NH3 •The red represents areas of positive charge, the blue the negative charge •In CH4, the red positive charge is evenly distributed throughout the molecule indicating a nonpolar molecule. •In NH3, the negative and positive charges are separated from each other indicating a polar molecule. Methods of Telling Polarity (4.12) •A quick way to determine something about polarity of a molecule is to: 1. Draw the Lewis structure 2. If the central atom has any nonbonded pairs around it, it will be a polar molecule. 3. If the central atom has only atoms around it and they are all the same, it is a nonpolar molecule. 4. If the central atom has only atoms around it and any of them are different, it is a polar molecule. • On the previous slide, CH4 has four atoms all the same around it – it is nonpolar. • NH3 has a nonbonded pair of electrons and will be polar • These guidelines are general. Sometimes electronegativity differences will be small enough that a molecule, even with different atoms around the central atom, will be virtually nonpolar. An example of this is compounds containing only carbon and hydrogen – these are considered nonpolar regardless of geometry. • There are also molecules with 5, 6, or 7 atoms and/or nonbonded electrons bonded to the central atom. Those don’t strictly follow the rules above. Those we won’t worry about in this course. A Simulation •If you would like to get some practice on molecule polarity using the PHET simulations, go to: •http://phet.colorado.edu •Click on the chemistry icons in the upper left. •Choose the Molecule Polarity simulation •You can Save It and then run it or Run it Now •A reasonable way of using this simulation is to focus simply on changing the electronegativity of the atoms and watching what happens to the arrow. The arrow represents what we call the dipole moment and points toward the negative charge in the molecule. •Take a look at the 2-atom, 3-atom, and molecule tabs to get a feel for how the polarity idea works. Importance of Polarity (4.12) •Properties of compounds depend to a large extent on the polarity or nonpolarity of the compound. Properties affected include: •boiling point •melting point •vapor pressure •solubility •Some of these properties and their relationship to polarity will be explored later.


![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)