Academie Ste. Cecile International School SCH3U – Chemistry 11
advertisement

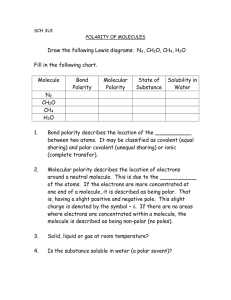
Academie Ste. Cecile International School SCH3U – Chemistry 11 Practice Problems – Predicting the polarity of a molecule Name: Date: Part 1 – Questions 1. What is a polar covalent bond and which types of atoms form them when bonded together? 2. If a molecule contains a polar covalent bond does that make the entire molecule polar explain? 3. What is the main difference between a polar molecule and a non polar molecule? Part 2 – Predicting Polarity Instruction – By Building the following molecules and then drawing the appropriate Lewis dot diagram and structural diagram you will predict the polarity of each of the molecules. Make sure you fully label the dipoles on each atom and show the flow of electrons in each bond for the structural diagram. Molecule Urea – H2NCONH2 Formaldehyde – CH2O Hydrogen Cyanide – HCN Chloroflorocarbon – CCl2F2 Amino Acid – H2NCOOH Lewis Dot Diagram Structural Diagram Trimethylamine – (CH3)3N Ethanol – C2H5OH Ethanoic acid (Vinegar) – H3CCOOH Methyl Ether – CH3OCH3 Acetone – CH3COH Octane – C8H18 Prop – 1 – ene – CH3CHCH2 Chloral Hydrate – Cl3CCH(OH)2 Lactic Acid – H3CCH(OH)COOH