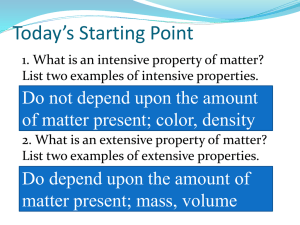
Physical Science Unit 2 Test Read through the questions carefully and read through each multiple choice you are given before making your decision. DO NOT WRITE ON THIS EXAM. Bubble in your Gradecam ID on the scantron sheet before beginning. 1. What is the title of Unit 2? a. Nature of Science b. Classification of Matter c. The Atomic Structure 2. Matter can be classified by it’s physical state which are: a. Solids liquids and gasses b. Pure substances and Mixtures c. Elements and Compounds d. Homogeneous and Heterogeneous mixtures 3. In which state do the particles of matter slide past each and move freely? a. Solid b. Liquid c. Gas 4. In which state do the particles of matter hold a definite shape and volume? a. Solid b. Liquid c. Gas 5. In which state do the particles of matter have the most amount of energy? a. Solid b. Liquid c. Gas 6. Matter can be classified by it’s chemical composition which are: a. Solids, liquids, and gases b. Pure substances and Mixtures c. Elements and compounds d. Homogeneous and heterogeneous mixtures 7. A Pure substance can be classified into a. Elements and compounds b. Homogeneous and heterogeneous 8. Which of the following is incorrect? a. A pure substance is made of one kind of substance b. A pure substance can be broken up into smaller substance c. A pure substance can be either an element or a compound d. A pure substance’s smallest unit is called an Atom 9. Which of the following is incorrect? a. Anything that is matter has mass and volume b. Mass is the weight of an object c. Volume is how much space something takes up 10. A mixture is made of two or more substances that can a. Not be easily be separated b. Are chemically combined c. Are physically combined 11. In the flow chart diagram, what was the example that was given for a pure substance? a. pizza b. aluminum foil c. water 12. Mixtures can be classified into two categories: homogeneous and heterogeneous. Which of the following statements is true? a. The root word homo means “different” b. The root word hetero means “same” c. A homogeneous mixture looks the same throughout d. A heterogeneous mixture looks the same throughout 13. Pure substances can be classified into two categories: elements and compounds. Which of the following statements is true? a. Elements can be broken down into smaller substances b. The smallest unit of an element is called a molecule c. Compounds are combinations of elements 14. Mrs. Conrad asked you to attach your periodic table on a. Page 38 b. Page 44 c. The back cover of your notebook d. The front cover of your notebook 15. Chemical symbols represent the elements on the periodic table. Which of the following statements are true? a. A chemical symbol is written using all capital letters b. A chemical symbol is written using all lower case letters c. A chemical symbol is written using one capital letter and/or one capital letter followed by a lower case letter 16. What is the chemical symbol for Sodium? a. So b. Na c. Sd d. NA 17. What is the chemical symbol for Copper? a. Co b. C c. Cu d. Cp 18. What is the chemical symbol for Chlorine? a. Cl b. Ci c. CL d. Ch 19. What is the chemical symbol for Iron? a. I b. Ir c. Fr d. Fe 20. On page 45 you drew a graph representing the distribution of elements found in our bodies. What kind of graph did you draw? a. A table chart b. A pie chart c. A line graph d. A bar graph For Questions 21 through 35, Classify each of the pictures by using the following answer key: A= Element D = Mixture of Compounds B = Compound E = Mixture of Elements and Compounds C= Mixture of Elements 21. ___________ 22._____________ 23.______________ 24._____________ 25.______________ 26.______________ 27.____________ 28._______________ 29.________________ 30.___________ 31.___________ 32.______________ 33.____________ 34.___________ 35._____________ 36. How much water is in our bodies? a. 65% b. 18% c. 10% d. 75% 37. A subscript number is the large number written in the front a chemical formula to represent the number of molecules a. true b. false 38. NaCl is an example of: a. An element which is an atom b. An element which is a molecule c. A compound which is an atom d. A compound which is a molecule 39. O2 is an example of: a. An element which is an atom b. An element which is a molecule c. A compound which is an atom d. A compound which is a molecule 40. In the chemical formula NH4NO3, what is the number of different elements? a. 4 b. 3 c. 9 41. In the chemical formula C4H8FCOOH, what is the number of different elements? a. 7 b. 4 c. 17 42. In the chemical formula Mg(NO3)2, how many atoms of Nitrogen are there? a. 1 b. 2 c. 3 d. 4 43. In the chemical formula Zn(OH)2, how many atoms of Zinc are there? a. 1 b. 2 c. 3 d. 4 44. In the chemical formula Cr(NH3)6(NO3)3, what is the total number of atoms? e. 5 f. 25 g. 37 h. 41 45. If Gatorade is considered a solution, what would be it’s Solute? i. The powder j. The water 46. I was riding my bike and I got a flat. Inflating my bike tire would be a k. A physical change l. A chemical change 47. Turning an ice cube into water is called Melting. This would be a a. Physical change b. Chemical change 48. Two substances are mixed and light is produced. This would be a a. Physical change b. Chemical change Look at the diagram to answer the following questions: 49. Which point represents the Melting Point? a. A b. B c. C d. D 50. Which point represents the Boiling Point? a. A b. B c. C d. D 51. During the slopes identified with the state of matter, what is happening to the Kinetic Energy? a. Increasing b. Decreasing c. Staying the same 52. During the slopes identified with the state of matter, what is happening to the Potential Energy? a. Increasing b. Decreasing c. Staying the same 53. During phase change, the temperature is a. Increasing b. Decreasing c. Staying the same 54. During phase change, the Kinetic energy and Potential energy are a. Increasing b. Decreasing c. Staying the same