Matter Matters - Fall River Public Schools
advertisement

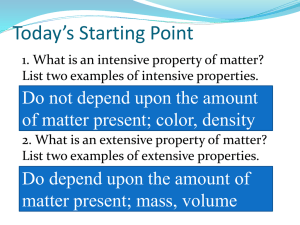
Chemistry Ms. Piela A property of a substance that can be measured or observed without changing its composition ◦ Examples Size/Shape Density Mass Melting point/Boiling point/ Freezing point Any change that occurs to a substance that does not change its chemical composition ◦ Examples Cutting Breaking Melting Boiling Intensive: does NOT depend on how much substance there is ◦ Density ◦ Color Extensive: DOES depend on how much substance there is ◦ Weight ◦ Length Example ◦ Which of the following are physical changes? (Circle all that apply) a) Making caramel from sugar b) Carving a wooden figure c) Freezing mercury d) dissolving salt in water A property of a substance that can be measured or observed and changes its composition ◦ Examples Reactivity Water Oxygen pH For chemical changes to occur, a chemical reaction must take place Chemical reactions are the rearrangement of atoms and molecules that forms new substances Example: CH4 + 2 O2 → CO2 + 2 H2O Demo Example: Burning Magnesium 2 Mg + O2 → 2 MgO ◦ Reactants are the initial substances before a reaction ◦ Products are substance formed in a reaction Chemical change is any change in chemical composition that forms a new substance ◦ Examples Rusting Decomposing compost Evolution of a gas Formation of a precipitate ◦ Precipitates are solids that form in a solution Release or absorption of energy ◦ i.e. heat released, light given off, sound Color change in a reaction system Practice Problem #1 ◦ Which of the following are chemical changes and which are physical changes? a) Cracking an egg b)Removing a stain with bleach c) Burning a candle d)Melting butter Practice Problem #2 ◦ For the following, identify them as chemical property, chemical change, physical property, or physical change: a) Decomposition of mercury (II) oxide b) Iron reacts with oxygen, while gold does not c) Oxygen is colorless, odorless, and soluble (dissolves) in water d) Under extreme pressures, oxygen will liquefy Chemical Changes •Any change that forms a new substance •Example: Burning, Decomposing Physical Changes •Any change that does not change the chemical makeup of the substance •Example: Cutting, Melting What is matter? ◦ Anything that has mass and takes up space ◦ Examples: Desks Air Lazy Students Insane Science Teachers Solids ◦ have definite shape and definite volume Example Diamonds ◦ Particles are slow and will vibrate in place Liquids ◦ have indefinite shape and definite volume Example Water ◦ Particles move faster and slide past one another Gases ◦ Have indefinite shape and indefinite volume ◦ Example Air ◦ Particles move fast with large spaces in between Solids Liquids Gases Definite Shape Indefinite Shape Indefinite Shape Definite Volume Definite Volume Indefinite Volume Particles vibrate in place Particles slide past one another Particles move freely Matter Pure Substances Element Compound Mixtures Homogeneous Heterogeneous Pure substances are substances that contain only one kind of matter ◦ Example Water (H2O), Oxygen (O2), Iron (Fe) Mixtures are physical blends of one or more pure substances ◦ Example Salad, Soup Matter Pure Substances Element Compound Mixture Homogeneous Heterogeneous Homogeneous mixtures are mixtures with uniform composition ◦ Examples: Milk, Gas, Air Heterogeneous mixtures are mixtures that do not have uniform composition ◦ Examples: Salad, Soup Matter Pure Substances Element Compound Mixture Homogeneous Heterogeneous Example Problem ◦ Determine whether the following examples are heterogeneous mixtures or homogeneous mixtures a) Stainless steel b) Italian Dressing c) Maple syrup d) Chocolate chip cookie Elements are the simplest form of matter ◦ Examples: Carbon (C), Gold (Au), Mercury (Hg) Compounds are two or more elements chemically combined ◦ Examples: Water (H2O), Sugar (C6H12O6) (not O2, N2) Matter Pure Substances Element Compound Mixture Homogeneous Heterogeneous Practice #1 a) b) c) d) e) f) CH4 Compound S8 Element Concrete Heterogeneous Distilled water Compound Salt water Homogeneous CH2O Compound Practice #2 ◦ Mixture. A physical blend of two substances Practice #3 ◦ Blue solid is compound. Can’t determine others