Practice problems - Bio-Link
advertisement

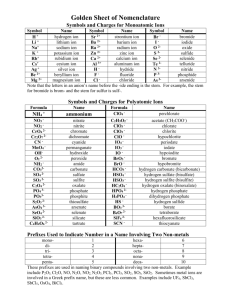
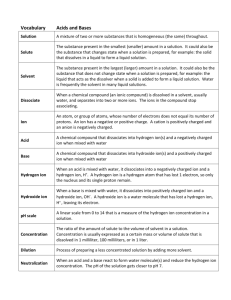
1 LOGARITHMS, ANTI-LOGARITHMS, AND pH Practice Problems Point 1: The common log (logarithm) of a number is the power to which 10 must be raised to give that number: 100 = 102 The log of 100 is 2. Point 2: An anti-log (antilogarithm) is the number corresponding to a given logarithm: The antilog of 2 = 100 Because 10 raised to the second power equals 100 Point 3: pH is a method to express the concentration of hydrogen ions in a solution. This concentration typically varies between 1.0 M and 1 X 10 –14 M. pH is defined as the negative logarithm of the hydrogen ion concentration. If a solution has a hydrogen ion concentration of 0.01 M, the pH of that solution is 2. Why? 0.01 M = 1 X 10–2 M The log of 10–2 = -2. pH = -log[H+] Therefore, the pH = -(-2) = 2. 2 Self-Test: Please complete the following: 1. What is the pH of a solution with a hydrogen ion concentration of 0.001 M? 2. What is the pH of a solution with a hydrogen ion concentration of 0.003 M? 3. What is the pH of a solution with a hydrogen ion concentration of 2 X 10-1 M? 4. What is the hydrogen ion concentration of a solution with a pH of 2? 5. What is the hydrogen ion concentration of a solution with a pH of 3? 6. What is the hydrogen ion concentration of a solution with a pH of 2.5? Complete the following: 1. log 3 = ____________ 2. log 10 = ___________ 3. log 0.1 = ___________ 4. antilog 2 = ________ 5. antilog 3 = __________ 6. antilog 2.5 = ___________ 3 Without using a calculator, explain how you know that the following is incorrect. 1. log 50 = 3.2 2. antilog 4.5 = 123,456