Chapter 4: Acids, Bases, and Buffers
advertisement

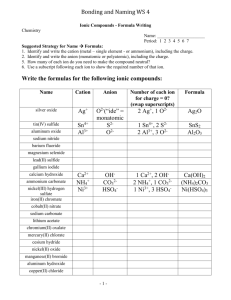
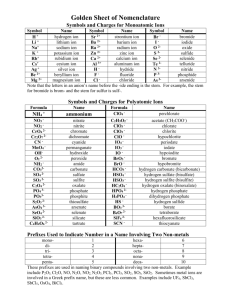
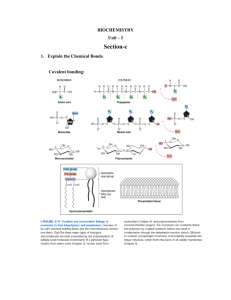
Chapter 4: Acids, Bases, and Buffers Objective Shortcut: 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Keywords, Assignments, Practice Questions, Other Help, Home Page Objectives: 1. Recognize a hydrogen bond and atoms or groups of atoms that can take part in forming a hydrogen bond. Top 2. Define the ion product for water! Given any hydroxide ion concentration, determine the hydrogen ion concentration and visa versa. Top 3. Define pH. Given any hydrogen ion concentration, determine the pH and visa versa Top 4. Define an acid and a base. Top 5. Recognize the acids produced by the body during metabolism and be able to state the predominant form at pH = 7.4 (ic or ate, ammonium or ammonia). Top The “predominant form” will be covered later in the chapter. 6. What is the difference between a strong acid and a week acid? Can a strong acid serve as a buffer? Top 7. Given a weak acid, be able to draw the equation for its dissociation and label the conjugate base (salt of the acid). Be able to define the Ka for the acid. Be able to write the HendersonHasselbalch equation for an acid. Top 8. Define the term buffer! Name two factors that determine the effectiveness of the buffer! At what pH are buffers most effective? Top 9. What is the most important reason for maintaining a physiological pH? Top 10. What are the two most important buffers in blood? 11. Be able to draw the equations that show how the bicarbonate buffer system works in blood! What is the respiratory compensation when the blood pH drops to 7.3? What is the respiratory compensation when the blood pH rises to 7.5? Top 12. Draw the dissociation of ammonium ion. If the pKa =9.3, what form is found at pH = 7? Top 13. Regarding Di Abietes: Define Type 1 diabetes. With out insulin, what happens to the blood glucagon concentration? What happens to her blood glucose and ketone body levels (concentrations)? Top 14. Regarding Di Abietes: Explain why an increase in a metabolic acid would cause the changes seen in PaCO2 and serum bicarbonate. What would happen to the concentration of CO2 and serum bicarbonate after the insulin injection? Top 15. Regarding any person suspected of having diabetes: What levels of fasting plasma glucose or levels of random plasma glucose would you expect to measure? Top 16. Concerning Dennis Veere: If the pKa for acetylsalicylic acid to acetylsalicylate is 3.5, is aspirin a weak or strong acid? Which form is prevalent in the stomach at a pH of 1? Which form is prevalent in blood at pH of 7.4? Prove it using the Henderson-Hasselbalch equation. Top 17. Regarding Percy Veere: Using the Henderson-Hasselbalch equation, explain the effect of breathing into a paper bag upon blood pH. Top Keywords: acetic acid, acetoacetic acid, acetylsalicylic acid, ammonia, ammonium ion, beta-hydroxybutyric acid, bicarbonate, carbon dioxide, carbonic acid, citric acid, conjugate base, dissociation constant, glucagon, hemoglobin, Henderson-Hasselbalch Equation, hydrogen bond, insulin, ion product, Ka, pKa, ketone bodies, ketoacidosis, Kussmaul’s respiration, lactic acid, metabolic acids, metabolic bases, pH, phosphoric acid, respiratory alkalosis, respiratory compensation, strong acid, sulfuric acid, weak acid. Top Assignments: Understand the meaning of the key words in the context of Chapter 4. Examine Questions (Q:)and Answers(A:) in Chapter 4. Work Review Questions 1, 3, 4, and 5 at the end of the Chapter 4. Not Question 2. Note that the answers to these Review questions are in the Appendix.