Ph Laboratory Exercise - SCF Faculty Site Homepage
advertisement

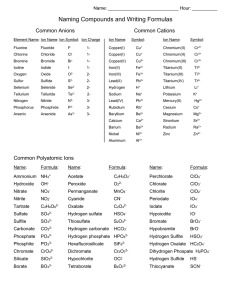
pH Laboratory Exercise Lab lecture Pre lab assignment- Visit the following web site and print out the procedure http://www.middleschoolscience.com/cabbage.htm PH paper will be used in place of the red and blue litmus paper. Visit the following web address for background material http://www.chem.ubc.ca/courseware/pH/index.html I. pH basics A. Def- A measurement of hydrogen ion (hydronium ion) concentration in a given solution. B. pH= - log [H+], H+ + H2O H3O+ Where [ ] indicate concentration in moles/ liter 1. Review the concept of a mole 2. review metric units of measurement C. Acids are defined as proton donors 1. Hydrogen ion concentration increases as a result of the dissociation of a proton from molecule containing hydrogen. 2. The increase in hydrogen ion concentration results in a drop in the pH due to the inverse relationship between the two quantities. 3. Changes of 1 pH unit indicate 10 fold changes in hydrogen ion concentration. 4. Acids have a pH below 7 in aqueous solution B. Bases are defined as proton acceptors. 1. Basic substances are anionic 2. Strong bases usually dissociate into a cation and a hydroxide ion (OH-) 3. Bases have a pH above 7 in aqueous solution C. Pure H2O is neutral 1. H2O H+ + OH2. [H+] = 10-7 D. Neutralization reaction 1. Mixture of an acid and a base to achieve a pH of 7 2. Can be used to determine the molar quantities of an unknown acid or base if the exact concentration of the opposite agent is known. a. Titration b. End point c. Equivalency E. Buffer 1. Def- A substance that resists changes in pH 2. Usually consists of a weak acid and its anionic conjugate base 3. Relies on shifts in equilibrium in reversible dissociation/association reactions 4. CO2 + H2O H2CO3H+ + HCO35. Titration curve depicts pH range over which a buffer is most effective 6. Each buffer system is most effective at pH’s surrounding the dissociation constant for the weak acid. 7. Henderson Hasselbach equation 8. pH = pKa + log [base]/[acid] 9. The ratio of base to acid determines the exact pH of the buffer solution II Biological significance A. Homeostatic parameter for all living organisms B. Specific biochemical components of living organisms display pH optimums 1. Enzymes 2. All H bonded macromolecules III. Lab procedures A. Follow procedures outlined in the procedure page web address above with noted changes. B. Construct a table of pH values for the substances tested using the pH paper. C. Construct a graph of the color displayed by the cabbage juice indicator vs. the pH determined by pH paper or the electronic probe (if available) D. Discuss possible mechanisms for antacid medications. E. Report 1. A standard report must be written by each student using the form provided. 2. The report must contain a table of pH values and a graph of color vs. pH as discussed above 3. Possible mechanisms for antacid chemistry must be included along with supporting scientific argument.