Name Chemical Compounds 1. What is the correct chemical formula
advertisement

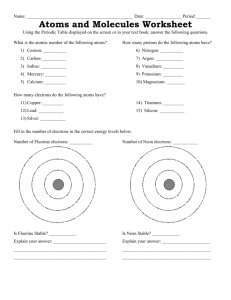
Name ______________________________ Chemical Compounds 1. What is the correct chemical formula for sodium carbonate? A. Na2CO3 B. NaCO3 C. Na(CO3)2 2. What is the correct chemical formula for calcium nitrate? A. CaNO3 B. Ca(NO3)2 C. Ca(2NO3) D. Ca2NO3 3. The physical properties of water are A. very similar to the physical properties of both hydrogen and oxygen B. very different from the physical properties of hydrogen or oxygen C. more like hydrogen than oxygen because water has more hydrogen D. more similar to oxygen because most of the mass of water comes from oxygen 4. How do the properties of a compound compare to the elements that form it? A. Compounds always have properties very similar to the properties of the individual elements from which they are formed. B. Compounds often have properties which are very different from the properties of the individual elements from which they are formed. 5. Which of the following statements about hydrogen peroxide (H2O2) and water (H2O) correctly describes their properties? A. Their properties are similar because they are made of the same elements. B. Their properties are different because the elements are present in different ratios. 6. Carbon monoxide (CO) and carbon dioxide (CO2) are both produced when fossil fuels are burned. Carbon monoxide is deadly and is responsible for deaths in the US every year. Carbon dioxide is not. Which statement best explains why? A. Combining elements in different proportions produces compounds with different properties. B. Elements are harmful when they are combined with only one oxygen. C. The properties of a compound are predicted by the elements that compose them. 7. How many valence electrons does an atom in Group 2, period 4 have? A. 2 B. 4 C. 8 D. 14 8. Use a periodic table to answer this question. How many valence electrons does chlorine have? A. 2 B. 5 C. 7 D. 9 9. If an oxygen atom gains 2 electrons to form a stable molecule it becomes an ion with a charge of A. -2 B. +2 C. -4 D. +4 10. An atom that loses 3 electrons will have which charge? A. 0 B. 3 C. +3 D. -3