Acids and Bases - Cloudfront.net
advertisement

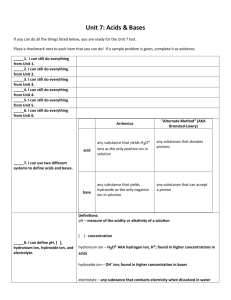
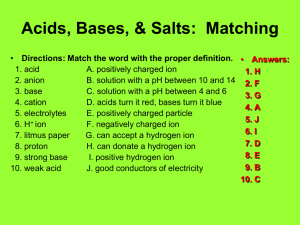
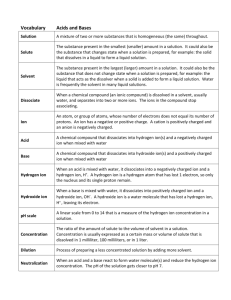
Acids and Bases Acids are substances that have a pH below 7 on the pH scale. They are made up of a positively charged ion and a negatively charged ion. The positively charged ion within an acid is usually the hydrogen ion (H+). The pH scale measures the amount of H+ ions in a substance. The hydrogen ion, H+, is a positively charged atom created through the loss of one electron. Bases are substances that have a pH higher than 7 on the pH scale. Group 7A elements on the periodic table form negatively charged ions, such as Cl- and have a -1 charge. They gain one electron to make them negative.When these negative ions bond to H+ ions, they form the strong acids HCl, HF, HI and HBr. The name for HCl Hydrogen Chloride. The names for the ions contained within the acids are the names of the elements. The -ine is dropped from the name of the negative ion and -ide is added. The H+ ion also combines with the OH- ion to form H-O-H or water. Water is a neutral compound with the pH of 7. Water, since it is a polar molecule, can act as a solvent for an acid. Water molecules can dilute acids by pulling acid molecules away from each other and also separating acid molecules into their ions. One reaction that can occur is the formation of the H3O+ ion when an acid is mixed with water. The H3O+ ion indicates the presence of a weak acid. The chemical equation for this reaction is: H-A + H2O ------> H3O+ + OH Knowledge and Comprehension Words to Know: 1. Which group of elements usually pairs with H+ to make acids? 2. What happen to an acid when it mixes with water? Application, Analysis, Evaluation, and Synthesis 3. Indicate the name of the following strong acids: Formula Name HF - ____________________________ HCL - ___________________________ HBr - ___________________________ HI - ____________________________ 4. Describe how the H30+ ion is created. 5. What is the difference between an acid and a base. 6. Explain how water can act as a solvent in the presence of an acid. Acid: Base: pH Scale: Neutral compound: 7. In terms of pH, explain how a strong acid and water are different.