Recall: What exactly is a bond? Depends*Ionic or Covalent? Polar?
advertisement
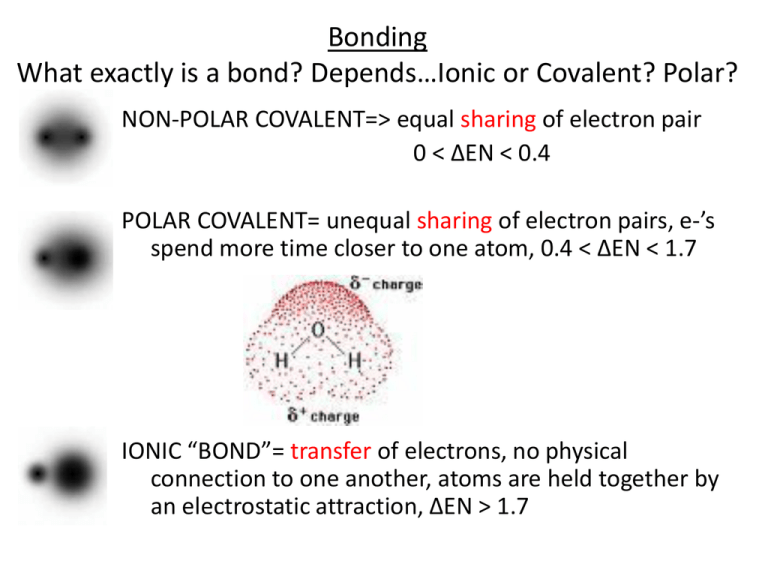
Bonding What exactly is a bond? Depends…Ionic or Covalent? Polar? NON-POLAR COVALENT=> equal sharing of electron pair 0 < ∆EN < 0.4 POLAR COVALENT= unequal sharing of electron pairs, e-’s spend more time closer to one atom, 0.4 < ∆EN < 1.7 IONIC “BOND”= transfer of electrons, no physical connection to one another, atoms are held together by an electrostatic attraction, ∆EN > 1.7 Molecular Polarity • • • • Tutorial 1: p. 226 Figure 6: p. 228 p. 227 #1,2 HW: p. 229 #1-7ab No molecular dipole => non-polar molecule Molecular Dipole is present => molecule No polar molecular dipole => non-polar molecule Molecular Dipole is present => polar molecule Which process requires more energy? Why? H2O(l) H2O(g) or 2 H2O(l) 2 H2(g) + O2(g) Intermolecular Forces London force (dispersion) •due to electrostatic attraction b/w protons in one molecule and electrons of neighbouring molecules •strength α # of eCl2 bp = -35°C Cl2 has 34 e-. I2 has 106 e-. I2 bp = 84°C I2 has more e- => greater LDF => Explain the trend in bp. higher bp Intermolecular Forces dipole-dipole force •due to attraction of one dipole by surrounding dipoles •strength α molecular polarity CH2O bp = -21°C C2H6 bp = -89°C Explain the trend in bp. Include diagrams to support your explanation. CH2O is a polar molecule => has a dipole force, LDF (16 e-) C2H6 is a non-polar molecule => no dipole force, only LDF (18 e-) CH2O has higher bp due to dipole-dipole force Predict which substance has the stronger dipole force: HCl or HBr HCl is the more polar molecule (ΔEN is greater) => stronger dipole forces (Draw diagrams to support your explanation.) Intermolecular Forces Hydrogen bonding •due to attraction of a H bonded to a highly EN atom (O, N or F) in one molecule by the lone pair of e- on a highly electronegative atom of a neighbouring molecule H2Te bp = -10°C H2Se bp = -50°C H2S bp = -80°C H2O bp = 100°C Explain the trend in bp. Intermolecular Forces Identify the type of IMF Strongest? Properties of Liquids Cohesive forces: • attractions b/w like molecules Adhesive forces: • attractions b/w unlike molecules Capillary Action • Water is transported in thin tubes from roots to shoots. • adhesive forces (b/w H2O and sides of tubes) and cohesive forces (b/w H2O molecules) • Water is pulled up against gravity! Intermolecular Forces and Properties i) Boiling and melting pt ii) Surface tension iii) Meniscus shape iv) Capillary action v) Volatility vi) Viscosity vii) Solubility viii) Wetting Action ix) Hydrophobicity Why is glycerol more viscous than water? Homework • Section 4.7 • p. 244 #1,2 • p. 247 #1-6






