Bibliographic Information The action of certain esters and ethers of
advertisement

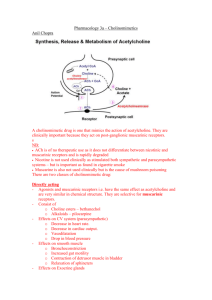
Bibliographic Information The action of certain esters and ethers of choline, and their relation to muscarine. Dale, H. H.. London, J. Pharmacol (1914), 6 147-90. Journal language unavailable. CAN 9:633 AN 1915:633 CAPLUS Abstract In the action of choline, and, with varying degrees of intensity, in the action of its esters (marked with acetylcholine, choline nitrous ester and choline nitric ester) and certain ethers (choline ethyl ether), 2 distinct types of action can be detected-a "muscarine" action, paralyzed by atropine, and a "nicotine" action, paralyzed by excess of nicotine. The action of choline nitrous ether is identical with "synthetic muscarine." Acetylcholine occurs occasionally in ergot, but its instability renders it improbable that its occurrence has any therapeutic significance. It appears to be hydrolyzed in the blood. Muscarine • Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. It was first isolated from Amanita muscaria in 1869. It was the first parasympathomimetic substance ever studied and causes profound activation of the peripheral parasympathetic nervous system that may end in convulsions and death. Muscarine has no effects on the central nervous system because it does not cross the blood-brain barrier due to its positively charged (polar) nitrogen atom.Muscarine mimics the action of the neurotransmitter acetylcholine at metabotropic receptors that are also known under the name muscarinic acetylcholine receptors.Muscarine poisoning is characterized by increased salivation, sweating (perspiration), and tearflow (lacrimation) within 15 to 30 minutes after ingestion of the mushroom. With large doses, these symptoms may be followed by abdominal pain, severe nausea, diarrhea, blurred vision, and labored breathing. Intoxication generally subsides within 2 hours. Death is rare, but may result from cardiac or respiratory failure in severe cases. The specific antidote is atropine.Muscarine is only a trace compound in the fly agaric Amanita muscaria; the pharmacologically more relevant compound from this mushroom is muscimol. Nicotine • Nicotine is an alkaloid found in the nightshade family of plants (Solanaceae), predominantly in tobacco, and in lower quantities in tomato, potato, eggplant (aubergine), and green pepper. Nicotine alkaloids are also found in the leaves of the coca plant. Nicotine constitutes 0.3 to 5% of the tobacco plant by dry weight, with biosynthesis taking place in the roots, and accumulates in the leaves. It is a potent neurotoxin with particular specificity to insects; therefore nicotine was widely used as an insecticide in the past, and currently nicotine derivatives such as imidacloprid continue to be widely used.In lower concentrations (an average cigarette yields about 1mg of absorbed nicotine), the substance acts as a stimulant in mammals and is one of the main factors responsible for the dependence-forming properties of tobacco smoking. According to the American Heart Association, "Nicotine addiction has historically been one of the hardest addictions to break. Some definitions • Para- (prefix): A prefix with many meanings, including: alongside of, beside, near, resembling, beyond, apart from, and abnormal.For example, the parathyroid glands are called "para-thyroid" because they are adjacent to the thyroid. For another example, paraumbilical means alongside the umbilicus (the belly button).The prefix "para-" comes straight from the Greek. Methacholine • Metacholine (also spelt methacholine) is a synthetic choline ester that acts as a non-selective muscarinic receptor agonist in the parasympathetic nervous system. It is highly active at all of the muscarinic receptors, but has little effect on the nicotinic receptors. Metacholine has a charged quaternary amine structure, rendering it insoluble to lipid cell membranes. Clinically, this means that it will not cross the blood-brain barrier and has poor absorption from the gastrointestinal tract. It is broken down at a relatively slow rate within the body, due to its resistance to acetylcholinesterases.The primary clinical use of methacholine is to diagnose bronchial hyperreactivity, which occurs in asthma. This is accomplished through the metacholine challenge test. Other therapeutic uses are limited by its adverse cardiovascular effects, such as bradycardia and hypotension, which arise from its function as a cholinomimetic.Use of metacholine, as well as all other muscarinic receptor agonists, is contraindicated in patients with coronary insufficiency, gastroduodenal ulcers, and incontinence. The parasympathomimetic action of this drug will exacerbate the symptoms of these disorders. Carbachol • Carbachol (Kar-ba-kol key), also known as carbamylcholine (marketed under the brand names Carbastatィ, Carbopticィ, Isopto Carbacholィ, Miostatィ), is classified as a cholinergic. Thus, it acts as an AChR agonist. It is primarily used for various ophthalmic purposes, such as for treating glaucoma, or for use during ophthalmic surgery. It is generally administered as an intraocular solution (i.e. eyedrop). • Carbachol is a choline ester and a positively charged quaternary ammonium compound. It is not well absorbed in the gastro-intestinal tract and does not cross the blood-brain barrier. It is usually administered topical ocular or through intraocular injection. Carbachol is not easily metabolized by cholinesterase, its duration of action is 4 to 8 hours with topical administration and 24 hours for intraocular administration. Since carbachol is poorly absorbed through topical administration, benzalkonium chloride is mixed in to promote absorption.Carbachol is a parasympathomimetic that stimulates both muscarinic and nicotinic receptors. In topical ocular and intraocular administration its principal effects are miosis and increased aqueous humour outflow.In the cat and rat, carbachol is well-known for its ability to induce rapid eye movement (REM) sleep when microinjected into the pontine reticular formation. Carbachol elicits this REM sleep-like state via activation of postsynaptic muscarinic cholinergic receptors (mAChRs).[edit]IndicationsCarbachol is primarily used in the treatment of glaucoma, but it is also used during ophthalmic surgery. Carbachol eyedrops are used to decrease the pressure in the eye for people with glaucoma. It is sometimes used to constrict the pupils during cataract surgery.Topical occular administration is used to decrease intraocular pressure in people with primary open-angle glaucoma. Intraocular administration is used to produce miosis after lens implantation during cataract surgery. Carbachol can also be used to stimulate bladder emptying if the normal emptying mechanism is not working properly.In most countries carbachol is only available by prescription. Definitions • A parasympathomimetic is a drug or poison that acts by stimulating or mimicking the parasympathetic nervous system (PNS). These chemicals are also called cholinergics because acetylcholine (ACh) is the neurotransmitter used by the PNS. • Chemicals in this family can act by either directly stimulating the nicotinic or muscarinic receptors, or they can act indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. • Some Chemical weapons such as sarin or VX, Non-lethal riot control agents such as tear gas, and insecticides such as Diazinon fall into this category. Definitions • Sympathetic and parasympathetic divisions typically function in opposition to each other. But this opposition is better termed complementary in nature rather than antagonistic. For an analogy, one may think of the sympathetic division as the accelerator and the parasympathetic division as the brake. • The sympathetic division typically functions in actions requiring quick responses. • The parasympathetic division functions with actions that do not require immediate reaction. • Consider sympathetic as "fight or flight" and parasympathetic as "rest and digest". 1. Nerve Transmission Peripheral nervous system Skeletal muscle CNS (Somatic) Ach (N) CNS (Autonomic) Synapse Ach (N) NA Sympathetic Adrenaline Parasympathetic Ach (N) Adrenal medulla AUTONOMIC Synapse Ach (N) Ach (M) Smooth muscle Cardiac muscle Bethanechol • Bethanechol (be-Than-e-kol [key]) is a parasympathomimetic choline ester that selectively stimulates muscarinic receptors without any effect on nicotinic receptors. Its chemical structure is 2-((aminocarbonyl)oxy)N,N,N-trimethyl-1-propanaminium. Unlike acetylcholine, bethanechol is not hydrolyzed by cholinesterase and will therefore have a long duration of action.Bethanechol is sometimes given orally or subcutaneously to treat urinary retention resulting from general anesthetic or diabetic neuropathy of the bladder, or to treat gastrointestinal atony (lack of muscular tone). The muscarinic receptors in the bladder and gastrointestinal tract stimulate contraction of the bladder and expulsion of urine, and increased gastrointestinal motility, respectively. Bethanechol should be used to treat these disorders only after obstruction is ruled out as a possible cause.Use of bethanechol, as well as all other muscarinic receptor agonists, is contraindicated in patients with asthma, coronary insufficiency, peptic ulcers, and incontinence. The parasympathomimetic action of this drug will exacerbate the symptoms of these disorders.Bethanechol is sold under the brand names Duvoid (Roberts), Myotonachol (Glenwood), and Urecholine (Merck Frosst). Medicinal Importance of Ach Agonists • • • • • • Types of direct acting muscarinic receptor agonists:a) Esters of choline (eg. acetylcholine, pilocarpine, carbachol, bethanechol chloride)-poorly absorbed, -variable susceptibility to hydrolysis by cholinesterase -affects duration of actionb) Alkaloids (eg. muscarine)-well absorbed, not used clinically -mushroom poisoning (Amanita muscaria)i) Acetylcholine-highly susceptible to hydrolysis -IV bolus lasts 5-20 seconds -limited use in topical application in ophthalmologyii) Pilocarpine-acts on smooth muscles of eye to constrict the pupil (miosis) used to treat glaucoma -contracts ciliary muscles by stimulating muscarinic receptors -rapid penetration (15-30 min) and long duration (8 hrs) increased aqueous outflowiii) Carbachol-carbamyl ester of choline -used mainly in ophthalmology for cataract surgery (causes rapid miosis) -decreases intraocular pressure by opening drainage angle of anterior chamber of eye - .: used in glaucoma (when resistant to pilocarpine or physostigmine) Bethanechol Chloride-choline ester -persistent effects because it is resistant to cholinesterases -selectively stimulates urinary and gastrointestinal tracts -facilitates emptying of neurogenic bladder in patients after surgery or parturition or with spinal cord injury O O N N pilocarpine • Pilocarpine has been used in the treatment of chronic open-angle glaucoma and acute angle-closure glaucoma for over 100 years.[1] It acts on a subtype of muscarinic receptor (M3) found on the iris sphincter muscle, causing the muscle to contract and produce miosis. This opens the trabecular meshwork through increased tension on the scleral spur. This action facilitates the rate that aqueous humor leaves the eye to decrease intraocular pressure.Pilocarpine is also used to treat dry mouth (xerostomia). Pilocarpine stimulates the secretion of large amounts of saliva and sweat. It may also cause hypotension and bradycardia in the cardiovascular system, and bronchospasm and increased bronchial secretion in the lungs due to its nonselective muscarinic action. O O N N pilocarpine •Pilocarpine is used to stimulate sweat glands in a sweat test to measure the concentration of chloride and sodium that is excreted in sweat. It is used to diagnose cystic fibrosis (CF). •Pilocarpine is available under several trade names such as: Diocarpine (Dioptic), Isopto Carpine (Alcon), Miocarpine (CIBA Vision), Ocusert Pilo-20 and -40 (Alza), Pilopine HS (Alcon), Salagen (Pharmacia & Upjohn), Scheinpharm Pilocarpine (Schein Pharmaceutical), and Timpilo (Merck Frosst). •Adverse effectsUse of pilocarpine may result in a range of adverse effects, most of them related to its action as a muscarinic receptor agonist. Pilocarpine has been known to cause excessive sweating, excessive salivation, bronchospasm, increased bronchial mucus secretion, bradycardia, hypotension, and diarrhea.The therapeutic uses of pilocarpine are limited by its adverse effects. Sweat Testing for Cystic Fibrosis From Stacey Lloyd, Diagnosing Cystic Fibrosis with Sweat Testing For the past forty plus years, sweat testing has been the most effective, and therefore the most popular, test for detecting cystic fibrosis. However, if a person tests positive for cystic fibrosis, a repeat of the sweat test should be performed to confirm the diagnosis. How Sweat Testing is Performed The sweat test is performed by using an electrode filled with pilocarpine. The electrode is placed on the inner forearm, and a second electrode is placed on the outer forearm. A current is run through the electrodes delivering the pilocarpine under the skin. Sweating testing is painless, however the person will feel a slight tingling sensation on the skin where the eletrodes are placed. The electrodes are removed and the arm is left to rest for about a one-half hour with the filter paper. The filter paper will soak up any sweat released from the person's skin. Once the one-half hour is up, the filter paper is placed into a flask and rinsed to release the sweat from the filter. A digital chloridometer is used to measure the concentration of chloride ions in the fluid. If more than 60 mmol/liter is detected, cystic fibrosis is diagnosed. Quick Fact: Many, many years ago, doctors would lick babies shortly after birth to determine if they had cystic fibrosis! Cystic fibrosis Cystic fibrosis (CF) is the most common fatal genetic disease in the United States today. It causes the body to produce a thick, sticky mucus that clogs the lungs, leading to infection, and blocks the pancreas, stopping digestive enzymes from reaching the intestines where they are required to digest food. CF is caused by a defective gene, which codes for a chloride transporter found on the surface of the epithelial cells that line the lungs and other organs. Several hundred mutations have been found in this gene, all of which result in defective transport of chloride, and secondarily sodium, by epithelial cells. As a result, the amount of sodium chloride (salt) is increased in bodily secretions. The severity of the disease symptoms of CF is directly related to the characteristic effects of the particular mutation(s) that have been inherited by the sufferer. CF research has accelerated sharply since the discovery of CFTR in 1989. In 1990, scientists successfully cloned the normal gene and added it to CF cells in the laboratory, which corrected the defective chloride transport mechanism. This technique—gene therapy—was then tried on a limited number of CF patients. However, this treatment may not be as successful as originally hoped. Further research will be required before gene therapy, and other experimental treatments, prove useful in combating CF.