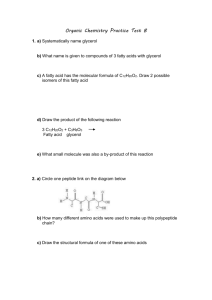
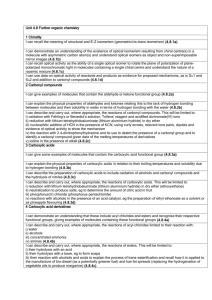
File
advertisement

Latasha Tanner, Christopher Waters, Olivia Dubberly, Kristen Long, Jon Canalizo Team Activity 9 In your Teams, prepare 3 multiple choice questions on the topic of Buffers and pH. 1) If you have two buffer solutions, one with a concentration of 0.2 M and the other with a concentration of 0.01M which one would be closer to the theoretical pH value? a. The 0.2 M solution b. The 0.01 M solution c. There is not enough information d. They will both be the same 2) Which of the following would have a better buffer capacity? a. 0.5 M acid and 0.5 M base b. 0.25 M acid and 0.3 M base c. 1.0 M acid and 0.95 M base d. 0.01 M acid and 0.02 M base 3) In order to create a buffer at pH 7.3 from a weak acid with a pKa of 7.7 what ratio of acid to conjugate base would you use? a. 11.4:1 b. 0.4:1 c. 2.5:1 d. 8.9:1 In your Teams, prepare 3 multiple choice questions on the topic of Solubility. 1) Which of the following causes an increase in solubility in a polar solvent of a compound? a. Higher melting point b. Increasing number of carbons c. More polar groups on the molecule d. Having a trans isomer 2) If the solubility pH of a weak acid is 9.5 and you increase the pH, will a precipitate form? a. Yes, a precipitate will form. b. No, a precipitate will not form. 3) Which one of these molecules is more soluble? a. b. c. d. all the same Latasha Tanner, Christopher Waters, Olivia Dubberly, Kristen Long, Jon Canalizo In your Teams, list 5 drugs from your “Top 200 Prescription Drug List” from PHA 371 – Practice of Pharmacy that are weak acids and 5 drugs from this list that are weak bases. Identify the weak acid or weak base functional group in each molecule. Weak Acid Amoxicillin dehydrate – the carboxylic acid group Naproxen – the carboxylic acid group Gabapentin – the carboxylic acid group Niacin – the carboxylic acid group Singular – the carboxylic acid group Weak Base Omperazole – the carbonyl group Hydrochlorthiazide – the multiple carbonyl groups Amlodipine besylate – the amine groups Metformin – the amine groups Spiralactone – the carbonyl groups