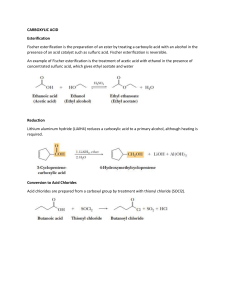
Chemistry 2213: Visual Cheat Sheet for Carboxylic Acids and Derivatives _____________________________________________________________________________ Purpose - This document contains the reactions related to the unit “Carboxylic acids” and “Derivatives of carboxylic acids” How to use this review - Before the exam, we recommend that you attempt to familiarize yourself with all the reactions covered in the Organic Chemistry 2213 course. This document is a visual cheat sheet summarizing the reaction details and electron movements for the reactions taught in the last two units of the course. Most of the reactions will have supplementary notes to describe in-detail the specifics of the reaction. We wish you the best of luck on your exams! Disclaimer: This visual assumes that you have thoroughly reviewed your material. This cheat sheet is a way for us to provide all the mechanisms in one area so you can avoid flipping through your notes. Stay up to date with what WebStraw has to offer. This resource was made by the resource design team at WebStraw. We are a student operated non-profit organization dedicated to providing open-access, high quality educational resources to university students. To be notified when similar resources like this are released, subscribe to our email list here(we promise we won’t spam you). 1 Carboxylic Reactions Acid-Base Reactions Note: ● Production of a weaker base and acid ● Equilibrium reaction, does not react all the way Reduction of Primary Alcohols Note: ● LiAlH4 is a strong reducing agent 2 Decarboxylation of beta-keto acids Note: ● Carbonyl must be in the beta position Decarboxylation of beta dicarboxylic acid 3 Fischer Esterification Mechanism: Note: ● Acid Dependant Carbonyl Activation ● Nucleophilic Attack on carbocation carbon ● H2O leaving Group Ejection by Oxygen “Nucleophile” 4 Lactone Formation (Cyclic Esterification) Acid-Chloride Formation Mechanism: 5 Acid Derivative Reactions Anhydride Hydrolysis Note: ● 2nd most reactive ● Anhydrides also react rapidly with water, but slower than acid halides. No catalyst is needed, but one can be helpful ● Result is a carboxylic acid byproduct 6 Anhydride Hydrolysis: Carboxylation Ester Hydrolysis: Carboxylation Note: ● ● ● ● Require catalyst: heat + acid or base Tetrahedral carbonyl addition intermediate formed In base: product is carboxylic acid salt + alcohol ○ Saponification, provides good yields because salt precipitates In acid: product is carboxylic acid + alcohol 7 Amide Hydrolysis: Carboxylation Note: ● ● ● Amides are very stable ○ Thus require higher temperature and stronger acid or base to hydrolyze In acid: liberated amine react with acid to form salt In base: base reacts with acid product to form salt 8 Conversion to other Acid Derivatives Acid chloride Note: ● Two moles are required 9 Esters to Amide: Amidification Note: ● ● ● ● Nucleophilic acyl substitution 2nd weakest acid derivative (only makes amides) Amine can be NH3, RNH2, or R2NH Need 2 equivalents of amine 10 Esters to Alcohols Note: ● 2 nucleophilic attacks for each Grignard reagent (carbonyl reforms) ● Produces 3° alcohol ● Stop after 1st step using Gilman reagent to make ketone Reduction of esters 11