Chemistry Midterm Exam Review - Matter and Change
advertisement

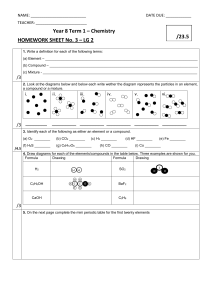
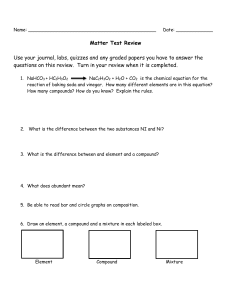
Chemistry Midterm Exam Review 2013 These are topics from a traditional 1st quarter. If you used the thematic approach with me this year, you will notice that the topics are not in order of their presentation throughout the year. The final exam is cumulative so this review will help you with reviewing the concepts and calculations. Notice that I have connected chapters to each unit from your book. Don’t forget the importance of the review sheet. Mid-Term Review I - Complete this page on Monday, March 18, 2013 Unit 1: Matter and Change (R= Ch 1&2 H= Ch 1 & 3) 1. Define chemistry. 2. State the difference between quantitative and qualitative data. 3. a. Define matter. b. Name the three states and list 2-3 characteristics for each of them. 4. What is the difference between a physical and chemical property? Give examples of each. 5. A chemical change is also known as a chemical _______________. 6. Name 5 buzz words that signify a physical change and 5 that signify a chemical change. 7. Define pure substances and state examples. 8. Define element and compound & state examples. 9. By what means can you separate a compound? Give some examples. 10. By what means can you separate a mixture? Give some examples. 11. What is the difference between a homogeneous and heterogeneous mixture? List some examples of each. 12. State whether each is a compound or element: Fe, CO, CaCl2, Hg, Co, argon, sodium chloride, I2. 13. Write the symbols for the following. mercury, gold, iodine, calcium, barium, tin, magnesium, phosphorus. 14. Name five indicators(observations) of a chemical reaction. 15. Define the words “reactant” and “product”. In a chemical equation, where are the reactants located? Where are the products located? What separates them from each other? 16. Classify each as a physical or chemical change: a. food spoiling b. water boils c. nail rusting d. baking bread e. sugar dissolving in water f. tarnishing silver