Practice Problems – Intro to Organic Chemistry
advertisement

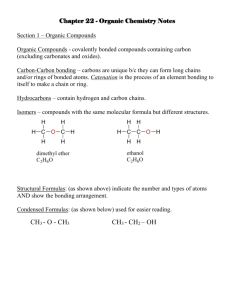
Name: __________________________________________________ Date: __________ Period: _____ Practice Problems – Intro to Organic Chemistry Part I: Multiple Choice. Circle the best answer to the question. 1(1). Which of the structures below is an aldehyde? O A. CH 3 CH 2CH O B. CH 3 CCH 3 C. (1) O CH 3 CH 2COH O D. CH 3 COCH3 2(7). Which of the substances below is least soluble in water? O A. CH2OHCHOHCH2OH C. CH 3 CH 2 COH O B. (1) O CH 3 CCH 3 D. CH 3 COCH 3 3(16). Which formulas represent butane or its isomer? I. II. III A. (1) CH3(CH2)2CH3 CH3CH(CH3)CH3 (CH3)3CH I and II only B. I and III only C. II and III only D. I, II and III 4(32). Which statement about neighboring members of all homologous series is correct? A. They have the same empirical formula. B. They differ by a CH2 group. C. They possess different functional groups. D. They differ in their degree of unsaturation. (1) 5(33). Which type of compound must contain a minimum of three carbon atoms? A. An aldehyde C. An ester B. A carboxylic acid D. A ketone (1) 6(34). What is the IUPAC name for CH3CH2CH(CH3)2? A. 1,1-dimethylpropane C. isopentane B. 2-methylbutane D. ethyldimethylmethane (1) 7(39). Which compound can exist as optical isomers? (1) A. B. H2NCH2COOH CH2ClCH2Cl C. D. CH3CHBrI HCOOCH3 8(48). Which compound has the lowest boiling point? A. B. CH3CH2CH(CH3)CH3 (CH3)4C 9(49). Which species will show optical activity? A. 1-chloropentane B. 3-chloropentane (1) C. D. CH3CH2CH2CH2CH3 CH3CH2OCH2CH3 C. D. 1-chloro-2-methylpentane 2-chloro-2-methylpentane (1) 1 Name: __________________________________________________ Date: __________ Period: _____ 10(63). Which compound is a member of the same homologous series as 1-chloropropane? A. 1-chloropropene C. 1-bromopropane B. 1-chlorobutane D. 1,1-dichloropropane (1) 11(64). Which formula is a correct representation of pentane? (1) A. B. CH3CH2CHCH2CH3 (CH3CH2)2CH3 C. D. CH3(CH2)3CH3 CH3(CH3)3CH3 12(74). Which formula is that of a secondary alcohol? A. B. CH3CH2CH2CH2OH CH3CHOHCH2CH3 C. D. (1) (CH3)2CHCH2OH (CH3)3COH Open Response – Answer the questions completely. Be sure to notice the point value and respond accordingly. Part II: 1(67). Give the structural formulas for the isomers of molecular formula C4H10 and state the name of each one. …………………………………………………………………………………………… …………………………………………………………………………………………… …………………………………………………………………………………………… …………………………………………………………………………………………… …………………………………………………………………………………………… …………………………………………………………………………………………… (Total 4 marks) 2(52). (a) (i) State and explain the trend in the boiling points of the first five alkanes. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) 3(ss). Which of the ONE pairs below best exhibits positional isomerism? A 4(ss). C D Which of the ONE pairs below best exhibits functional group isomerism? A 5(ss). B (2) B C (2) D Which of the ONE pairs below best exhibits hydrocarbon chain isomerism? A B C (2) D A. butane and 2-methyl propane B. butanal and 2-butanone C. 2-pentanol and 3-pentanol D. 2-pentene and cylopentane 2 Name: __________________________________________________ Date: __________ Period: _____ 6(14). A student prepared a sample of compound Y from benzene (C6H6) as follows: C6H6 → C6H5CH2CH3 → C6H5CHClCH3 X Y (b) (i) Draw two structures for compound Y, showing the relationship between them. (2) (ii) Explain the term plane-polarized light and describe how the optical isomers of Y could be distinguished using a polarimeter. ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ (2) (iii) Explain why a sample of Y produced in the lab might not show optical activity. ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ (4) 7(46). This question is based on the following reaction scheme. C5H10 → C5H11Br → C5H12O W X and Y Z (a) W has the structure (i) H 3C CH 2 CH 3 C H Give the structure of the geometrical isomer of W. C H (1) (ii) Explain why W has a geometrical isomer. (think! – what makes geometrical isomers possible?) .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... (2) (iii) State the full name of W. .......................................................................................................................... (2) 3 Name: __________________________________________________ Date: __________ Period: _____ SOME additional problems that rely on additional information… just to tempt you: (b) A compound C was soluble in water and on analysis was found to contain 60.0 % C, 13.3 % H and 26.7 % O by mass. The Mr of compound C is 60. (i) Calculate the empirical and molecular formulas of C. …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… (3) (ii) Draw three possible structural formulas for the isomers with this molecular formula. …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… (3) 28. Propene contains a C=C double bond whereas propanal contains a C=O double bond. (a) State and explain two similarities and two differences in the way in which the atoms are bonded in the covalent double bond in the two compounds. [hint – think about the bonds… what makes a double bond… think about structure, length, strength, polarity, etc.] Similarities: ................................................................................................................. ...................................................................................................................................... ...................................................................................................................................... ...................................................................................................................................... Differences: ................................................................................................................. ...................................................................................................................................... ...................................................................................................................................... ...................................................................................................................................... (4) 4 Name: __________________________________________________ Date: __________ Period: _____ Some helpful information …. (this won’t appear on the actual test ) Ok to pull this sheet off and keep it . # of C atoms 1 2 3 4 5 6 7 8 9 10 —Br, —Cl, —F, —I, —OH —NH2 Name as a substituent methyl ethyl propyl butyl pentyl hexyl heptyl octyl nonyl decyl bromo chloro fluoro iodo hydroxy amino phenyl Prefix for Main Chain meth eth prop but pent hex hept oct non dec Functional groups C–C C=C C=C COH CHO CCOC COOH COOC CON COC -O- Suffix for Main Chain -ane -ene -yne -ol -al -one -oic acid -oate -amide -ether or -oxy- Prefix for multiple substituents 2 3 4 di tri tetra 5 penta 5