Chapter 22 - Organic Chemistry Notes
advertisement

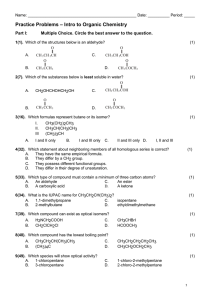
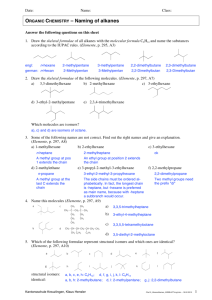
Chapter 22 - Organic Chemistry Notes Section 1 – Organic Compounds Organic Compounds - covalently bonded compounds containing carbon (excluding carbonates and oxides). Carbon-Carbon bonding – carbons are unique b/c they can form long chains and/or rings of bonded atoms. Catenation is the process of an element bonding to itself to make a chain or ring. Hydrocarbons – contain hydrogen and carbon chains. Isomers – compounds with the same molecular formula but different structures. Structural Formulas: (as shown above) indicate the number and types of atoms AND show the bonding arrangement. Condensed Formulas: (as shown below) used for easier reading. CH3 - O - CH3 CH3 - CH2 – OH Types of Isomers: 1. Structural Isomers (“Constitutional Isomers”) – atoms bonded together in a different order. 2. Geometric Isomers – bonded atoms are in the same order, but appear in different arrangements in 3D space. “cis” – odd atoms are on the SAME SIDE “trans” – odd atoms are on opposite sides Section 2: Hydrocarbons Saturated hydrocarbons – each carbon atom forms FOUR single bonds. Unsaturated hydrocarbons – not all carbon atoms have four single bonds. Hydrocarbon Description Alkane Contains only single bonds Example: (Saturated) General Formula CnH2n+2 Nomenclature Ends in –ane C3H8 C4H10 Propane Butane Examples Propane (above) Alkene Double bonds CnH2n Example: (Unsaturated) C3H6 C4H8 Ends in –ene Propene Butene Propene (above) Alkyne Triple bonds CnH2n-2 Example: (Unsaturated) C3H4 C4H6 Ends in –yne Propyne Butyne Propyne (above) Cycloalkanes or cycloalkenes: hydrocarbon in which the chain is connected in a ring…like Benzene (also known as cyclohexene). Carbon-Atom Chain Prefixes: 1 2 3 4 5 methethpropbutpent- 6 7 8 9 10 hexheptoctnondec- Nomenclature for Hydrocarbons: (p.721 – Honors; p._____) 1. Name parent hydrocarbon chain. a. Alkane – longest chain of single bonds. b. Alkene – If you have more than double bond, use suffixes to show how many and where the double bonds are located, 2=adiene, 3=atriene, 4=tetrene. c. Alkyne – If you have more than double bond, use suffixes to show how many and where the double bonds are located, 2=adiyne, 3=atriyne, 4=tetryne. 2. Add names of the branches a. If you have one branch, name it. b. If you have more than one branch of the same kind, use di=2, tri=3, tetra=4. 3. Number the carbons in parent chain. 4. Insert branch position numbers into the name. 5. Punctuate the name. Examples: CH3-CH-CH2-CH-CH-CH3 | | | CH3 CH3 CH3 CH3 | CH3-CH-C=CH2 | CH2-CH3 CH3≡≡C-CH-CH3 | CH3 Name: 2,3,5-trimethylhexane Name: 2-ethyl-3-methyl-1-butene Name: 3-methyl-1-butyne Properties & Uses Alkanes – nonpolar, weak London dispersion forces, as mass increases boiling point also increases, small molecules are gases, medium molecules are liquids, and large molecules are solids (waxes). Examples: Gasoline (octane). Alkenes – nonpolar, similar properties of boiling point and physical state as alkanes, used in commercial productions like making plastics and plant hormones like ethane (aka ethylene). Alkynes – nonpolar, same properties as above, used as ethyne (aka acetylene torches) for welding. Section 3 – Functional Groups Functional Group – group of atoms that give an organic compound its specific properties. List of functional groups you are responsible for knowing: Name Group Nomenclature 1. alcohol 2. alkyl halide 3. ether 4. 5. 6. 7. 8. 9. aldehyde ketone amine carboxylic acid ester thiol (-OH) ending in –ol (-F , -Cl , -Br , -I) use prefix (-O-) use ether use ___ oxy ___ (H-C=O) ending in –al (C=O) ending in –one (N) ending in –amine (-COOH) ending in –oic acid (O-C=O) ending in –oate (-SH) ending in –thiol See Attached Page… for more details! THE END! Example pentanol dichloropentane pentyl ethyl ether ethoxy pentane pentanal pentanone pentanamine pentanoic acid pentanoate pentane thiol