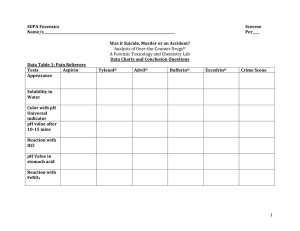
Acids, Bases, pH
advertisement
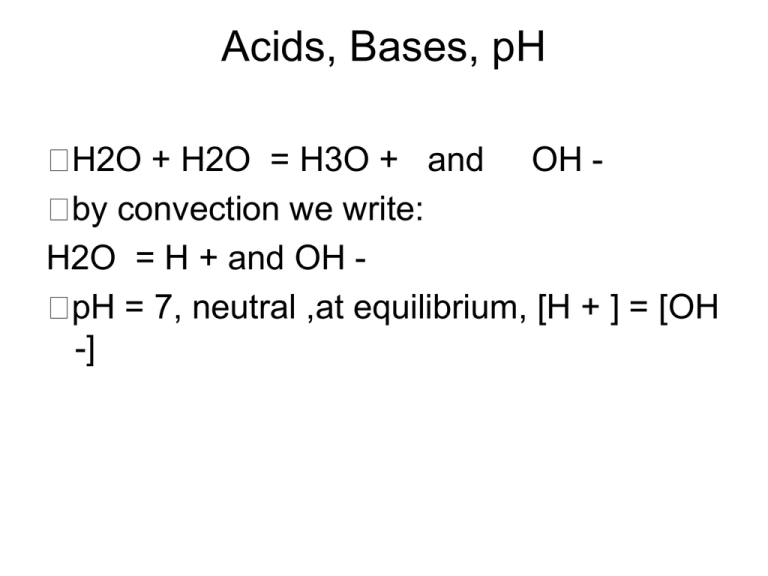
Acids, Bases, pH H2O + H2O = H3O + and OH by convection we write: H2O = H + and OH pH = 7, neutral ,at equilibrium, [H + ] = [OH -] pH Scale = 0-14 acid: dissociates H + , pH below 7, eg. HCl = H + and Cl base: dissociates OH - accepts H + ,pH above 7 ex. NaOH = Na + and OH ex. NH3 + H = NH4 Measuring pH using plant pigment 4.1 and 4.2 Anthocyanins = plant pigments in cabbage used as pH indicator eg red at low pH Procedure: make standards with the following pH buffers and record their color: 2, 4, 6, 7, 8, 10, 12. Compare unknown(4.2) Ex 4.3 The pH meter is more accurate than plant indicators. Procedure:Calibrate pH meter using pH 4 and pH 7 Determine pH of substances with pH meter and pH paper Buffers- 4.4 Buffer: prevents sudden pH changes Procedure:determine the buffering capacity of two substances (A & B) Follow the book instructions Graph your results for A & B Record which is the better buffer 4.5 How Effective are Stomach Antacids- Instructor demo Antacid: used to neutralize excess acid Procedure:ompare different antacids Rolaids, Tums, Alka-Seltzer determine effectiveness of antacids per gram( use 0.5 grams of each) run a control