Families on the Periodic Table
advertisement

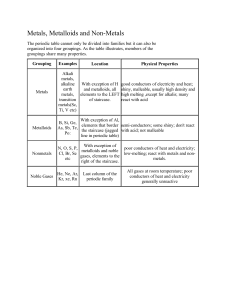
Families of the Periodic Table The Periodic Table Families of the Periodic Table: At the conclusion of our time together, you should be able to: 1. Name each region of the Periodic Table 2. Name each family of the Periodic Table 3. List at least 3 characteristics of each family of the Periodic Table Periods in the Periodic Table 1 2 3 4 5 6 7 Regions of the Periodic Table Metals Extra electrons Conductive Malleable Dense Shiny Ductile Metalloids Partly conductive Non-Metals missing electrons Non-Conductive NOT Malleable Dull Groups in the Periodic Table Elements in groups (families) react in similar ways! Hydrogen Elements Gas: Hydrogen Hydrogen (cont.) Characteristics Low density Low melting point Non-metal qualities: brittle, non-conductor, dull Reacts w/ halogens to form acids Reacts w/ oxygen to form water Group 1A: Alkali Metals Reaction of potassium + H2O Cutting sodium metal Alkali Metals Elements Solids: Lithium Potassium Sodium Rubidium Cesium Francium Alkali Metals (cont.) Characteristics Soft Dull silver color Low density Low melting point Metal qualities: shiny, good conductor, malleable, ductile Reacts w/ halogens to form salts Reacts w/ water to form strong alkaline hydroxides Group 2A: Alkaline Earth Metals Magnesium Magnesium oxide Alkaline Earth Metals Elements Solids Beryllium Magnesium Calcium Strontium Barium Radium Alkaline Earth Metals (cont.) Characteristics Soft Shiny silver color Low density Found naturally as a compound Metal qualities: shiny, good conductor, malleable, ductile Glow different colors Reacts w/ halogens to form ionic salts Transition Elements Lanthanides and Actinides (Inner Transitions) Iron in air gives iron(III) oxide Transition Metals Elements Solids All “D-block” elements Atomic numbers 21-30 39-48 57, 72-80 89, 104-112 Transition Metals (cont.) Characteristics High density High melting / boiling point Good catalysts Metal qualities: shiny, good conductor, malleable, ductile Hard, tough & strong Silvery blue at room temperature Multiple oxidation states Post-Transition Metals Elements Solids Aluminum Gallium Indium Tin Thallium Lead Bismuth Post Transition Metals (cont.) Characteristics Relatively high density Salts Solids Metal qualities: shiny, good conductor, malleable, ductile Not as reactive Opaque color Malleable Oxidation numbers +3, +4, +5 Metalloids Elements Solids Boron Silicon Germanium Arsenic Antimony Tellurium Polonium Metalloids (cont.) Characteristics Some shiny Some dull Some malleable, some not Some ductile, some not Has properties of both metals and nonmetals Various oxidation numbers Other Non-Metals Gases (Hydrogen) Nitrogen Oxygen Solids Phosphorus Sulfur Selenium Carbon Other Non-Metals (cont.) Characteristics Non-metal qualities: dull, brittle, nonconductor, etc. High ionization energy High electronegativity Gain electrons easily Various oxidation numbers Group 7A: The Halogens (salt makers) F, Cl, Br, I, At Halogens Solids Iodine Astatine Fluorine Chlorine Liquids Bromine Gases Halogens (cont.) Characteristics Only group in all 3 states of matter Highly reactive w/ alkali metals & alkaline earth metals High electronegativity Non-metal qualities: dull, brittle, non-conductor, etc. Noble (inert) Gases Group #8 atoms P shell full Very non-reactive VERY happy Group 8A: The Noble (Inert) Gases He, Ne, Ar, Kr, Xe, Rn Lighter than air balloons “Neon” signs Very Unreactive because they have full electron levels XeOF4 Noble Gases Elements Gases Helium Neon Argon Krypton Xenon Radon Noble Gases (cont.) Characteristics Non-reactive Odorless Tasteless Colorless Nonflammable Non-metal qualities: dull, brittle, nonconductor, etc. Inner Transition Metals Elements Solids All “F-block” elements Atomic numbers 58-71 90-103 Inner Transition Metals (cont.) Characteristics Most are manmade High melting points Metal qualities: shiny, good conductor, malleable, ductile Reactivity varies Usually bonded to non-metals AKA: lanthanides & actinides New Elements Elements Atomic numbers 113 and on New Elements Characteristics All are manmade Some will have metal qualities: shiny, good conductor, malleable, ductile Some will have nonmetal qualities: dull, brittle, non-conductor, etc. Some will have qualities of both Families of the Periodic Table: Let’s see if you can: 1. Name each region of the Periodic Table 2. Name each family of the Periodic Table 3. List at least 3 characteristics of each family of the Periodic Table Which pair of elements is MOST similar? Ca and F Na and Cl Ne and Ar Li and H Copper is an element that is used in electrical wires. What is the smallest unit of copper that still maintains the characteristics of copper? the atom the electron the nucleus the proton The picture below shows three objects that can be classified in the same group. Which of the following statements is true for all three of these objects? They are metals. They rust rapidly. They weigh the same. They are the same color.