Regents Unit 13: Electrochemistry
advertisement

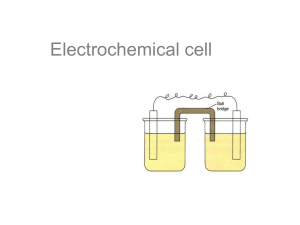
Utilizes relationship between chemical potential energy & electrical energy Redox Reactions • • • • Need battery to start car Prevent corrosion Bleach is an oxidizing agent Na, Al, Cl prepared or purified by redox reactions • Breathing – O2 H2O and CO2 Redox Reactions • Synthesis • Decomposition • Single Replacement Often Redox Always Redox • Double Replacement only is not redox Predicting Redox Reactions • Use Table J to predict if a given redox reaction will occur. • Any metal will donate its electrons to the ion of any metal below it. • Any nonmetal will steal electrons from the ion of any nonmetal below it. Predicting Single Replacement Redox Reactions • Element + Compound New Element + New Compound • If the element is above the swapable ion, the reaction is spontaneous. • If the element is below the swapable ion, the reaction is not spontaneous. Predicting Redox Reactions A + BX B + AX A & B are metals. If metal A is above metal B in Table J, the reaction is spontaneous. X + AY Y + AX • X & Y are nonmetals. If nonmetal X is above nonmetal Y in Table J, the reaction is spontaneous. Which are spontaneous? Yes Cs + CuCl2 Yes I2 + NaCl No Cl2 + KBr Yes Fe + CaBr2 No No Mg + Sr(NO3)2 F2 + MgCl2 Yes • Li + AlCl3 • • • • • • Started with Zn(NO3)2 & Cu and AgNO3 & Cu. Which beaker had the Zn ions & which had the Ag ions? Overview of Electrochemistry • TWO kinds of cells (kind of opposites): 1. Galvanic or Voltaic (NYS – Electrochemical) • Use a spontaneous reaction to produce a flow of electrons (electricity). Exothermic. 2. Electrolytic • Use a flow of electrons (electricity) to force a nonspontaneous reaction to occur. Endothermic. Vocabulary • • • • • • • Redox Half-reaction Oxidation Reduction Cell Half-Cell Electrode • • • • • • • Anode Cathode Galvanic Voltaic Electrochemical Electrolytic Salt bridge Electrochemical Cells • Use a spontaneous single replacement redox reaction to produce a flow of electrons. • Electrons flow from oxidized substance to reduced substance. • Called: Galvanic cells, voltaic cells, or electrochemical cells (NYS) Electrochemical Cells • Redox reaction is arranged so the electrons are forced to flow through a wire. • When the electrons travel through a wire, we can make them do work, like light a bulb or ring a buzzer. OJ clock • So the oxidation & reduction reactions have to be separated physically. Al / CuCl2 Lab • Was a redox reaction. • Did NOT force electrons to travel through a wire. Got NO useful work out of system. • Have to be clever in how we arrange things. 2Al + 3Cu+2 2Al+3 + 3Cu Got no useful work because halfreactions weren’t separated. Half-Cell • Where each of the half-reactions takes place. • Need 2 half-cells to have a complete redox reaction. • Need to be connected by a wire for the electrons to flow through. • Need to be connected by a salt bridge to maintain electrical neutrality. Schematic of Galvanic Cell Parts of a Voltaic Cell • 2 half-cells: oxidation & reduction • Each half-cell consists of a container of an aqueous solution & an electrode or surface at which the electron transfer takes place. • Wire connecting electrodes. • Salt bridge connects solutions. How much work can you get out of this reaction? • You can measure the voltage by making the electrons travel through a voltmeter. • The galvanic cell is a battery. Of course, it’s not a very easy battery to transport or use in real-life applications. Electrode Surface at which oxidation or reduction half-reaction occurs. Anode & Cathode An Ox Ate a Fat Red Cat • Anode – Oxidation • The anode = location for the oxidation half-reaction. • Reduction – Cathode • The cathode = location for the reduction half-reaction. Anode / Cathode • How do you know which electrode is which? • Use Table J to predict which electrode is the anode and which electrode is the cathode. Anode • Anode = Oxidation = Electron Donor • The anode is the metal that’s higher in Table J. Cathode • Cathode = Reduction = Electron Acceptor • The cathode is the metal that’s lower in Table J. Zn is above Cu, Zn is anode Notation for Cells ZnZn+2Cu+2Cu Direction of Electron Flow (wire) Anode to Cathode Direction of Positive Ion Flow (salt bridge) Anode to Cathode Positive & Negative Electrode • Negative electrode is where electrons originate – here it’s the Zn electrode. • Positive electrode is electrode that attracts electrons – here it’s the Cu electrode. Aqueous Solution • Solution containing ions of the same element as the electrode. • Cu electrode: solution may be Cu(NO3)3 or CuSO4. • Zn electrode: solution may be Zn(NO3)2 or ZnSO4. Salt Bridge • Allows for migration of ions between halfcells. • Necessary to maintain electrical neutrality. • Reaction will not proceed without salt bridge. A(s) + BX(aq) B(s) + AX(aq) • Single replacement rxn occurs during operation of galvanic cell. • One electrode will gain mass (B) and one electrode will dissolve (A). • The concentration of metal ions will increase in one solution (making AX) & decrease in one solution (using up BX). Half-Reactions Zn Zn+2 + 2eCu+2 + 2e- Cu _________________________ Zn + Cu+2 Zn+2 + Cu Which electrode is dissolving? Which species is getting more concentrated? Zn+2 Zn Zn + Cu+2 Zn+2 + Cu • Which electrode is gaining mass? Cu • Which species is getting more dilute? Cu+2 When the reaction reaches equilibrium • The voltage goes to 0. Construct Galvanic Cell with Al & Pb • Use Table J to identify anode & cathode. • Draw Cell, put in electrodes & solutions • Label anode, cathode, direction of electron flow in wire, direction of positive ion flow in salt bridge, positive electrode, negative electrode. • Negative electrode is where electrons originate. Positive electrode attracts electrons. Electron flow wire Al = anode - Al+3 Positive ion flow Salt bridge & NO3 -1 Pb = cathode Pb+2 & NO3-1 What are half-reactions? Al Al+3 + 3eAl metal is the electrode – it’s dissolving. Al+3 ions go into the solution. Pb+2 + 2e- Pb Pb+2 ions are in the solution. They pick up 2 electrons at the surface of the Pb electrode & plate out. Overall Rxn 2(Al Al+3 + 3e-) 3(Pb+2 + 2e- Pb) _____________________________ 2Al + 3Pb+2 2Al+3 + 3Pb 2Al + 3Pb+2 2Al+3 + 3Pb • • • • Al Which electrode is losing mass? Which electrode is gaining mass? Pb What’s happening to the [Al+3]? Increasing What’s happening to the [Pb+2]? Decreasing Application: Batteries Dry Cell Mercury battery Application: Corrosion Corrosion Prevention What’s wrong with this picture?