Aldehydes - ClassNet
advertisement

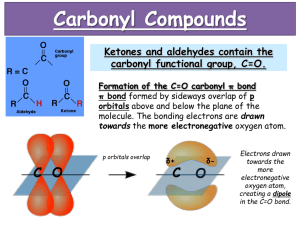
Aldehydes SCH 4UI Mr.Snyder Culminating Project Sherry Wong Aly Kadar Kasey Bourgon Define: Aldehydes An organic compound characterized by a terminal carbonyl functional group General Structure Functional group Terminal Carbonyl group One + H atom + (C=O) bonded to C R (Either another H atom or an alkyl group [carbon chain]) ∴ Carbonyl group always occurs at the end of a carbon chain Functional Group: Another H atom The H atom is attached to the C in the carbonyl group IUPAC: Methanal Common name: Formaldehyde Functional Group: Alkyl Group • Attached to the C in the carbonyl group o Examples 1. Cyclohexanal 2. IUPAC: Ethanal (Common Name: Acetaldehyde) General Structure Functional group Carbonyl group (C=O) + One H atom bonded to C + R (Either another H atom or an alkyl group [carbon chain]) ∴ Carbonyl group always occurs at the end of a carbon chain General Structure Difference between ALDEHYDES & KETONES Carbonyl Group Ketones Aldehydes Nomenclature: A Change of Suffix Ex: Methane -> Methanal (AKA: Formaldehyde) ★ Carbonyl group always at the end of the carbon chain ★ Replace the -ane at the end of the name with an -al ○ aldehyde Physical & Chemical Properties Boiling Point: Lower than analogous alcohols (ex. Ethanol - 78°C / Ethanal - 21°C) H-Bonds: Polarity: N/A (because there is no OH [hydroxide] groups) Strong polar group (due to double C=O bond) (electronegativity) Solubility: High Everyday Life Uses Smaller → Strong, unpleasant odours HYDROGEN ATOM Formaldehyde (aka. IUPAC name = Methanal) • • • • Simplest aldehyde Colourless gas @ room temperature In (aq) solution = antiseptic and disinfectant Used as a preservative ONE CARBON Acetaldehyde (aka. IUPAC name = Ethanal) • • Colourless liquid Synthesis of resins, dyes, and preservatives Trimer = 3 molecules joined together into a single large molecule Formaldehyde Fumigate rooms against pests Acetaldehyde Hypnotic drug Everyday Life Uses • • • Larger → Flowery, pleasant odours Ex. Cinnamaldehyde Found in essential oils of plants (ex. Frankincense egyptian and Rosalina Austrialian essential oils) Used for fragrance in perfumes and aromatherapy products o Aldehydes are refered to as aliphatic or "fatty" aldehydes o Fatty aldehydes have a chain of 8-13 carbon atoms (ex. Chanel No.5 Perfume) Reactions Kasey Reaction Worksheet Kasey Bibliography Kessel, H. v., Jenkins, F., Davies, L., Plumb, D., Giuseppe, M. D., & Lantz, O. (2003). Nelson Chemistry 12 . Toronto: Kevin Martindale. Aldehydes. (n.d.). perfume ingredient, fragrance and essential oils. Retrieved November 25, 2013, from http://www.fragrantica.com/notes/Aldehydes-165.html