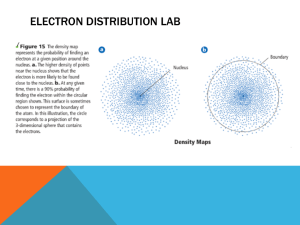
Where is the Electron Located?
advertisement

Where is the Electron Located? The Quantum Model of the Atom Heisenberg uncertainty principle: It is impossible to determine both the position and velocity of an electron or any other particle What is the Address of the Electron? Principle Quantum Number (n): Indicates the energy level occupied by an electron. Angular Momentum (l): Indicates the shape of the orbital (s,p,d,f,g) Atomic Numbers and Quantum Numbers Magnetic Quantum Number (m): Indicates the orientation of an orbital around the nucleus. Spin Quantum Number (↓↑): Indicates which way the electron is spinning What are the Rules Governing Electron Configuration? Aufbau Principle: An electron occupies the lowest energy orbital available Pauli Exclusion Principle: Only two electrons per orbital and they must spin in opposite directions Hund’s Rule: Each orbital of equal energy must have one electron before a second electron is added Let’s Fill Up The Orbitals! Summary of Orbitals Principle Sublevels Quantum # 1 s Number of Number of Orbitals Electrons 1 2 2 s, p 3 8 3 s, p, d 5 18 4 s, p, d, f 7 32 Predicting the 1s Orbital Exceptions to Aufbau



![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-300x300.png)