molar mass - AaronFreeman
advertisement

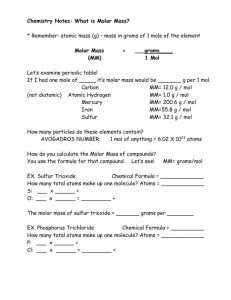
MOLAR MASS What is molar mass? How do you calculate molar mass? WHAT IS MOLAR MASS? • mass in grams of 1 mole of the element • Molar Mass (MM) = ___grams____ 1 Mol Synonyms: molecular weight, gram formula unit Use Periodic Table to determine MM! • Let’s examine periodic table! • If I had one mole of _____ it’s molar mass would be _______ g per 1 mol. • • • • • Carbon Atomic Hydrogen Mercury Iron Sulfur MM= 12.0 g / mol MM= 1.0 g / mol MM= 200.6 g / mol MM=55.8 g / mol MM= 32.1 g / mol How many particles do these elements contain? Particles: 1. Atoms 2. Formula Units 3. Molecules How do you calculate molar mass of compounds? • • • • • SO 3 EX. Sulfur Trioxide Chemical Formula = _____________ How many total atoms make up one molecule? 1+3=4 Atoms = _____________ 1 x ______ 32.1 = 32.1 S: ___ 48.0 + 16.0 = _____ 3 x ______ O: ___ 80.1 80.1 grams per • The molar mass of sulfur trioxide = _______ 1 mol ________ Your Turn PCl3 • EX. Phosphorus Trichloride Chemical Formula = __________ 1+3=4 • How many total atoms make up one molecule? Atoms = ________ 1 x ______ 31.0 31.0 = • P: ___ 3 x ______ 35.5 = _________ 106.5 + • Cl: ___ 137.5 grams per 1 mol • Sodium Hydrogen Carbonate NaHCO3 MM = 84.0 g / mol Representative Particles molecules • Covalent Compounds are made up of _____. Formula units • Ionic Compounds are made up of _________. atoms • Elements are made up of _____________. • Use your Road Map! Conversions Using Molar Mass • Book pg. 298 Al2O3 • Ex. #1 Write the chemical formula for aluminum oxide _________ • What is given? 9.45 moles Trying to find? g of Al2O3 moles Al2O3 x _______________ 102.9 grams Al2O3 = • 9.45 ______________ GIVEN 1 Mole Al2O3 Al: 2 x 27.0 = 54.0 g O: 3 x 16.0 = 48.0 g + 102.0 g / 1 mol 964 g of Al2O3 Molar Volume Conversions • At STP (Standard Temp and Pressure): • 1 mole of gas = 22.4 L = 6.02 x 1023 particles 1 Mole of ANY GAS at STP! • Sample Problem 10.7 • Sample Problem 10.8 Use our Mole Road Map! • REMEMBER! Density = mass = grams Volume Liter • HINT: If given density, first find the molar mass (MM) = grams per mol