Mole-Notes
advertisement

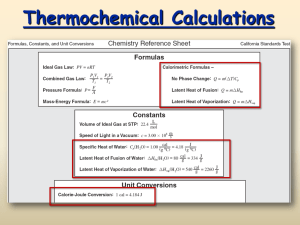
The Mole Meets Chem What is the Mole besides a furry rodent? 1. Unit of measurement indicating the amount of substance 2. Established by Amadeo Avogadro (1800's) 3. Amount of substance can be measured in terms of number of particles, mass or volume. Mole Troll The Molar Roads Gases 22.4L Volume The Mole Troll Bridge Riddle Me This: Are you a Gas? Are You at STP? Molar Mass Practice 1. 2,3-butene (12gx 4) + (1g x 6) = 54 g/mol 2. sodium pyrophosphate (23gx 4) + (31g x 2) + (16g x 7) = 266 g/mol 3. oxalic acid (12gx 2) + (1g x 2) + (16gx4) = 90g/mol 4. aluminum perchromate 5. C5H6 (27gx 2) + (52g x 3) + (16g x 15) = 450 g/mol (1gx 2) + (12g x 2) + (16g x 4) = 90 g/mol (12gx 5) + (1g x 6) = 66 g/mol Navigating the Molar Roads (Converting between molar mass, particles and volume with proportions) Given #,unit,substance = X (unknown), unit,substance Standard Value Standard Value (corresponds to given unit) (corresponds to unknown unit) Choices : 1 mol, molar mass, 22.4L, 6.02 x 1023 atoms,molecules,formula units (periodic table) Ex. How many moles are present in a 115g sample of cyclooctanol ?