What are atoms? Atoms are the basic building blocks of matter that
advertisement

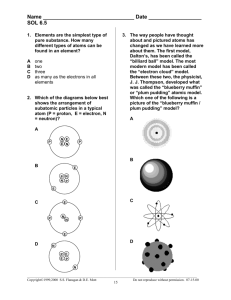
CH 4 ATOMIC STRUCTURE 1 WHAT IS IT AGAIN? An atom is a unit of matter, the smallest unit of an element that retains the chemical properties of that element. An element is a substance that cannot be broken down into simpler substances and retain the same qualities. Everything around us is composed of atoms. Your desk, the air, even you are made up of atoms! 2 HOW DO WE KNOW ANY OF THIS? Scientists came up with ideas and theories and performed experiments to test their ideas. No one scientist suddenly figured it all out-it took the work of many separate scientists working over a period of time to gather the knowledge that we have today! 3 DALTON’S THEORY (1803) 1)ALL ELEMENTS ARE COMPOSED OF ATOMS. 2)ALL ATOMS OF THE SAME ELEMENT HAVE THE SAME MASS, AND ATOMS OF DIFFERENT ELEMENTS HAVE DIFFERENT MASSES. 3)COMPOUNDS CONTAIN ATOMS OF MORE THAN ONE ELEMENT. 4)IN A PARTICULAR COMPOUND, ATOMS OF DIFFERENT ELEMENTS ALWAYS COMBINE IN THE SAME WAY. 4 DALTON’S THEORY (1803) A THEORY MUST EXPLAIN THE DATA FROM MANY EXPERIMENTS. BECAUSE DALTON’S ATOMIC THEORY MET THAT GOAL, THE THEORY BECAME WIDELY ACCEPTED. OVER TIME, SCIENTISTS FOUND THAT NOT ALL OF DALTON’S IDEAS ABOUT ATOMS WERE COMPLETELY CORRECT. THEY REVISED THE THEORY TO TAKE INTO ACCOUNT NEW DISCOVERIES. THOMSON’S MODEL OF THE ATOM (1897) When some materials are rubbed together, they gain the ability to attract or repel other materials. Such materials are said to have either a positive or a negative electric charge. • Objects with like charges repel, or push apart. • Objects with opposite charges attract, or pull together. 6 THOMSON’S MODEL OF THE ATOM (1897) Thomson revised Dalton’s model to account for newly discovered subatomic particles. • The atom has neither a positive nor a negative charge, but there must always be some positive charge in the atom. • The atom is filled with a positively charged mass of matter that has negative charges evenly scattered throughout it. 7 THOMSON’S MODEL OF THE ATOM (1897) Thomson’s model is called the “plum pudding” model. The negatively charged particles (electrons) are spread evenly through a mass of positively charged matter. 8 RUTHERFORD’S ATOMIC THEORY (1911) Ernest Rutherford designed an experiment to find out what happens to alpha particles (fast-moving, positively charged particles.) when they pass through a thin sheet of gold. Alpha particles He expected the particles to go straight through the gold foil… Thin Sheet of Gold WHAT ACTUALLY HAPPENED IN RUTHERFORD’S EXPERIMENT… Alpha particles Nucleus Rutherford proposed a new model. •The positive charge of an atom is not evenly spread throughout the atom. •Positive charge is concentrated in a very small, central area. •The nucleus of the atom is a dense, positively charged10 mass located in the center of the atom. BOHR’S MODEL OF THE ATOM (1913) Bohr’s atomic model had a nucleus surrounded by a large volume of space. But Bohr’s model focused on the electrons and their arrangement. In Bohr’s model, electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Each electron in an atom has a specific amount of energy. 11 WHAT ARE ATOMS MADE OF? Atoms are made up of three basic building blocks: Protons 2) Neutrons 3) Electrons 1) We know that these building blocks are made up of even smaller components called Elementary Particles, but we’ll worry about these later! 12 PROTONS, ELECTRONS AND NEUTRONS CAN BE DISTINGUISHED BY MASS, CHARGE, AND LOCATION IN AN ATOM. 13 Particle Charge Location Relative Mass Proton + nucleus 1 Neutron none nucleus 1 Electron - Electron cloud 1/1836 or 0.0005 14 Protons and Neutrons join together to form the Nucleus-the dense, positively charged central part of the atom Electrons are attracted to the protons in the nucleus by what is called the electromagnetic force. 15 USING THE PERIODIC TABLE … Atomic Number – Nitrogen Number of protons 7 N 14.007 Mass Number – Number of protons plus neutrons So … how would you figure out 16 how many neutrons an atom has? Using Nitrogen as our example… Mass Number – Atomic Number= Number of Neutrons 14.007 – 7 = 7.007 Neutrons Atomic Number – Nitrogen N 14.007 Number of protons 7 Mass Number – Number of protons plus neutrons 17 WHAT'S THE DEAL WITH THE .007? WHY ISN'T IT A WHOLE NUMBER? Atoms of the same element that have different numbers of neutrons are called Isotopes. Their Atomic number is the same but their Mass number changes Mass numbers are calculated by figuring out how many atoms of each type are out there in the universe. When you average out all of the masses, you get a number that isn’t quite a whole number Using Nitrogen as our example… -Most naturally occurring and stable Nitrogen atoms are N 14, but a few are N 15. Which is why our mass # is 14.007 18 WHAT ABOUT ELECTRONS? If the atom is neutral (does not have a + or – charge), then Electrons = Protons How many … Sodium 11 Na 22.99 1. Protons? 2. Neutrons? 3. Electrons? 19 http://www.miamisci.org/af/sln/phantom/spectroscope.html NUMBER OF ELECTRONS CONT. If the atom has a charge (ion)… Cations are atoms with a + charge Anions are atoms with a – charge Start with the number of electrons a neutral atom would have (same as # of protons) Then add electrons if it is a – charged anion Or subtract electrons if it is a + charged cation Example: Nitrogen with a +1 charge has _?_ electrons. 20 BOHR’S MODEL OF THE ATOM (1913) Energy Levels When an atom gains or loses energy, the energy of an electron can change. Electrons gain or lose energy when they move between fixed energy levels The possible energies that electrons in an atom can Nucleus have are called energy levels. • An electron cannot exist between energy levels. • Electron 21 Remember this guy…? -IN 1913 NIELS BOHR CAME UP WITH THE IDEA THAT ELECTRONS MOVED IN SPHERICAL ORBITS AROUND THE NUCLEUS. -THESE ELECTRONS MOVE IN ENERGY LEVELS THAT RADIATE OUT FROM THE NUCLEUS. -AN ELECTRON CAN MOVE FROM ONE ENERGY LEVEL TO ANOTHER WHEN THE ATOM GAINS OR LOSES ENERGY. 22 WE NOW KNOW THAT BOHR’S "SOLAR SYSTEM LIKE" PICTURE OF AN ATOM, WITH AN ELECTRON NEATLY MOVING AROUND A NUCLEUS IN A CIRCLE ISN'T REALLY CORRECT. 23 TURNS OUT, THERE'S NO REASON TO ASSUME THAT ELECTRON ORBITS ARE CIRCULAR. IN FACT IT'S VERY RARE FOR AN ATOM'S ELECTRON TO BE IN A CIRCULAR ORBIT. THE ELECTRON MOVES AT DIFFERENT SPEEDS. FAST NEAR THE NUCLEUS AND SLOW WHEN IT'S FAR FROM THE NUCLEUS. THE ELECTRON IS NOT ALWAYS THE SAME DISTANCE FROM THE NUCLEUS. SOMETIMES IT'S CLOSE, OTHER TIMES, IT'S FAR AWAY. 24 ELECTRON CLOUD MODEL…( BASED ON WORK BY AUSTRIAN PHYSICIST SHRODINGER IN 1924) An electron cloud is a visual model of the most likely locations for electrons in an atom. The cloud is denser at those locations where the probability of finding an electron is high. 25 ENERGY LEVELS CONTINUED… -We now know that the electrons don’t go around the nucleus in a neat circle, but we can still imagine distinct circular orbitals or energy levels within this electron cloud. -It is the electromagnetic force, or the attraction between the opposite charges (the positively charged proton and the negatively charged electron) that keep the electrons from flying off 26 Each of the orbitals can hold a fixed number of electrons The lower levels closer to the nucleus must be full before filling the upper levels. Chemical properties are based on the number of electrons in the outermost shell Elements with full outer shells are usually nonreactive Elements try to give up, take, or share electrons with the goal of having a full outer shell-these are more reactive elements 27