Atoms: The building blocks of Matter
advertisement

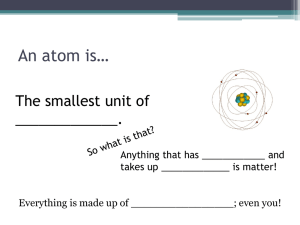
Atoms: The building blocks of Matter The Structure of the Atom Dalton’s Atomic Theory Democritus – atomos Lavoisier Law of Conservation of Matter Proust Law of Definite Proportions Dalton Atomic Theory Dalton’s Atomic Theory Questions: 1. What accounts for differences in properties between atoms of different elements? 2. What forces hold atoms together in compounds? Dalton’s Model: Hard, round object Discovery of the Electron Experiments by J.J. Thomson with cathode ray tubes showed that atoms contain electrons. (1897) J.J. Thomson: The Plum Pudding Model Atoms are mostly empty space. Millikan’s Oil Drop Experiment-1909 • Confirmed that electrons have a negative charge • The mass of an electron was determined (a small fraction of the mass of an atom). Two important inferences: 1. Atoms are neutral, so they must contain positive charge to balance the negatively charged electrons. 2. The small mass of electrons compared to atoms indicated that there must be other particles which account for an atom’s mass. Discovery of the Atomic Nucleus Rutherford’s Experiment (1911) Alpha Particle Scattering Experiment Expected results Actual Results Rutherford’s Conclusion • Atom’s contained a densely packed bundle of matter with a positive charge. • This bundle of matter was called the nucleus • Suggested that electrons move around the nucleus like planets around the sun Other Important Discoveries • (Mosely) Atoms of each element have a unique positive charge • Protons are positively charged particles in the nucleus • Neutrons are neutral particles in the nucleus • The charge of a proton is equal but opposite of the charge of an electron • Atoms contain an equal number of protons and electrons. • The # of protons in the nucleus gives each element its identity. • Strong nuclear forces hold protons and neutrons together in the nucleus