Unit 2 Self-Quiz, pages 270–271
advertisement

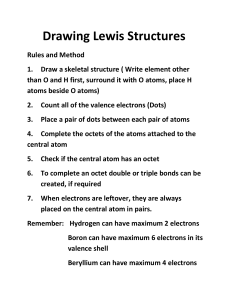
Unit 2 Self-Quiz, pages 270–271 1. (d) 2. (b) 3. (a) 4. (c) 5. (d) 6. (b) 7. (b) 8. (b) 9. (a) 10. (b) 11. (c) 12. (b) 13. (a) 14. (d) 15. (a) 16. (a) 17. (b) 18. (d) 19. (a) 20. (c) 21. (d) 22. (a) 23. (a) 24. (d) 25. False. The neutron is the most massive particle in the atom. 26. False. The neutron is an uncharged subatomic particle. 27. True 28. True 29. False. A line spectrum results when excited electrons emit energy. 30. True 31. False. John Dalton originated the idea that the atom is the smallest and indivisible unit of matter. 32. True 33. False. According to the Pauli exclusion principle, in a given atom, 2 electrons cannot share the same set of four quantum numbers. 34. True 35. False. Ionic compounds have electrostatic charge. 36. True 37. True 38. False. Carbon dioxide molecules have a linear shape. 39. False. In a polar covalent bond electrons are not shared equally between atoms. 40. True 41. True 42. True 43. False. Semiconductors are doped with atoms that have 1 more or 1 fewer valence electrons than the semiconductor atoms. Copyright © 2012 Nelson Education Ltd. Unit 2: Structure and Properties of Matter U2-10