Elements, Compounds & Reactions: Year 8 Science Presentation
advertisement
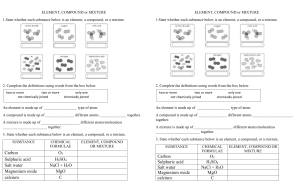
Year 8 Science 2013 Element A pure substance made up of only one type of atom it cannot be broken down into simpler substances by chemical reaction Compound A pure substance that contains two or more elements combined in a fixed ratio It can be broken down into its elements by chemical reactions Conservation of Matter The total mass of the reactants in a chemical reaction is always equal to the total mass of the products Matter cannot be created or destroyed Making and breaking compounds Sometimes atoms change the way they mix with each other. We call this a chemical reaction. Sometimes a chemical reaction creates by-products such as heat or light A chemical reaction can occur when: Lots of atoms or molecules join together to make one new compound. A compound breaks down into individual atoms. A few compounds mix up to make many new compounds Examples of chemical reactions Sodium Bicarbonate (Baking Soda ) + Hydrochloric Acid Sodium (Salt) + Water + Carbon Dioxide NaHCO3 + HCl NaCl + H2O + CO2 Magnesium + Oxygen Magnesium Oxide Mg2 + O2 2MgO Water Hydrogen + Oxygen 2H2O 2H2 + O2 Ion An atom that has a positive or negative charge Caused by the loss or gain of electrons Metals tend to lose electrons and nonmetals tend to gain electrons An atom that has gained an electron and an atom that has lost one are attracted to each other (like two ends of a magnet)