subtopic assessment (A)
advertisement
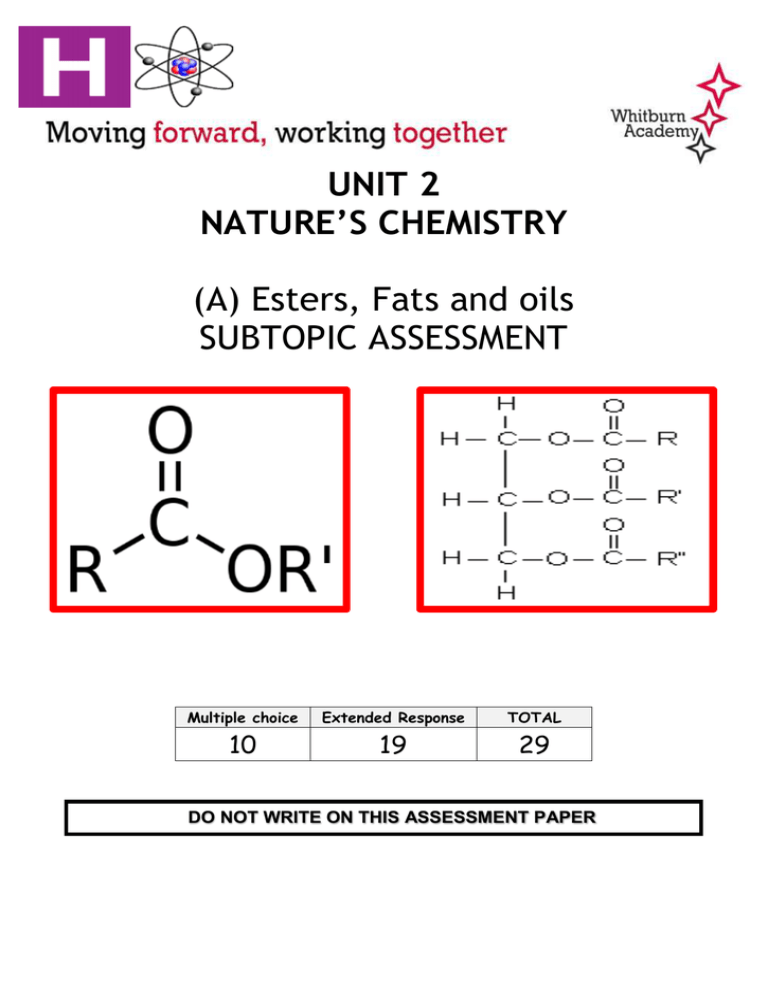
UNIT 2 NATURE’S CHEMISTRY (A) Esters, Fats and oils SUBTOPIC ASSESSMENT Multiple choice Extended Response TOTAL 10 19 29 DO NOT WRITE ON THIS ASSESSMENT PAPER Q1) Which of the following mixtures will form when NaOH(aq) is added to a mixture of propanol and ethanoic acid? A) B) C) D) Propanol and sodium ethanoate Ethanoic acid and sodium propanoate Sodium hydroxide and propyl ethanoate Sodium hydroxide and ethyl propanoate Q2) Oils contain carbon to carbon double bonds which can undergo addition reactions with iodine. The iodine number of an oil is the mass of iodine in grams that will react with 100g of oil. Which line in the table shows the oil that is likely to have the lowest melting point? Q3) When an oil is hydrolysed, which of the following molecules is always produced? Q4) Which of the following fatty acids is unsaturated? Q5) An ester has the following structural formula CH3CH2CH2COOCH2CH3 The name of the this ester is A) B) C) D) Propyl propanoate Ethyl butanoate Butyl ethanoate Ethyl propanoate Q6) An ester has the structural formula: On hydrolysis, the ester would produce A) B) C) D) Ethanoic acid and propan-1-ol Ethanoic acid and propan-2-ol Propanoic acid and propan-1-ol Propanoic acid and propan-2-ol Q7) Esters are formed by the reaction between which two functional groups? A) B) C) D) A hydroxyl group and a carboxyl group A hydroxyl group and a carbonyl group A hydroxide group and a carboxyl group A hydroxide group and a carbonyl group Q8) Oils are generally A) Solid at room temperature and contain a high proportion of unsaturated molecules. B) Solid at room temperature and contain a high proportion of saturated molecules. C) Liquid at room temperature and contain a high proportion of unsaturated molecules. D) Liquid at room temperature and contain a high proportion of saturated molecules. Q9) The antibiotic, erythromycin, has the following structure. To remove its bitter taste, the erythromycin is reacted to give the compound with the structure shown below. Which of the following types of compound has been reacted with erythromycin to produce this compound? A) B) C) D) Alcohol Aldehyde Carboxylic acid Ketone Q10) 4-Hydroxy-6-methyl-2-pyrone is a cyclic ester responsible for the smell of chocolate. The number 2 identifies the position of the carbonyl group in the pyrone ring counting from the oxygen atom within the ring. What is the structure of 4-hydroxy-6-methyl-2-pyrone? Extended Response Q1) A team of chemists is developing a fragrance for use in a shower gel for men. a) To give the gel a fruity smell the chemists are considering adding an ester. They synthesise six isomeric esters. Voluteers smell each ester and give it a rating out of one hundred depending on how fruity the smell is. i) Name the ester with the fruit-smell rating of 92. (1) ii) Shown below are the structure of three more isomers. (1) Q2) A calorie-free replacement for fat can be made by reacting fatty acids with the hydroxyl groups on a molecule of sucrose. A structural formula for sucrose is shown. How many fatty acid molecules can react with molecule of sucrose? (1) Q3) A student carried out some experiments using different fats and oils. a) The first experiment allowed the iodine number to be calculated. The iodine number is the mass of iodine, in grams, that will react with 100g of the fat or oil. The student’s results are shown. i) Shea butter is a solid at room temperature. Explain why the melting point of shea butter is higher than room temperature. (2) ii) Predict the iodine number of sunflower oil. (1) b) In the second experiment some oils were used to male soap. The oil, triolein, was reacted with sodium hydroxide. i) Name product X. ii) 5.0g of triolein was dissolved in ethanol and placed in a test tube with excess sodium hydroxide. The mixture was heated to 80°C. State a suitable method for heating the reaction mixture. iii) (1) (1) The experiment produced 1.28g of sodium oleate. Calculate the percentage yield. (2) Q4) Soft drinks contain many ingredients. Aspartame is added to many soft drinks as a sweetener. Its structure is shown below. Name the functional group circled. (1) Q5) Methanamide, HCONH2, is widely used in industry to make nitrogen compounds. It is also used as a solvent as it can dissolve ionic compounds. a) Why is methanamide a suitable solvent for ionic compounds? (1) b) In industry, mathanamide is produced by the reaction of an ester with ammonia. i) Name the ester used in the industrial manufacture of methanamide. (1) ii) Calculate the theoretical yield of mathanamide obtained if 100g of ammonia, NH3 is reacted with excess ester. (1) c) In the lab, methanamide can be prepared by the reaction of methanoic acid with ammonia. When 1.38g of methanoic acid was reacted with excess ammonia, 0.945g of methanamide was produced. Calculate the percentage yield of methanamide. Show your working clearly. (2) Q6) Asprin is a widely used medicine. It is advised that it is stored in dry, cool conditions. Using your knowledge of chemistry, comment on the reasons why asprin should be stored under these conditions. (3)