1. Ester, Fats and Oils Homework
advertisement
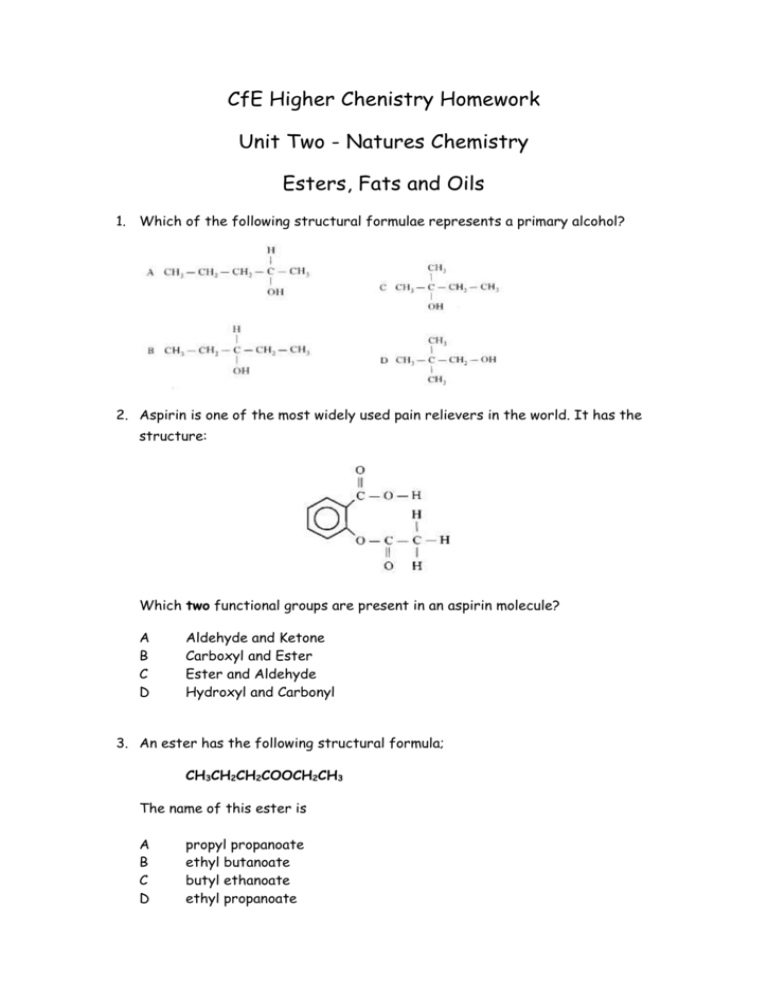
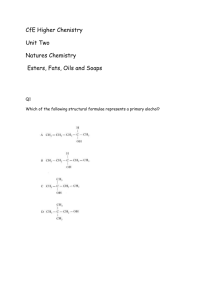
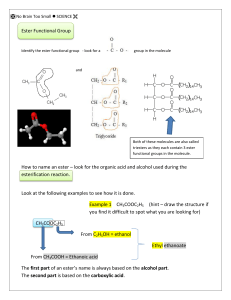
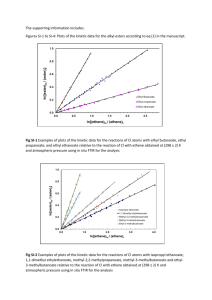
CfE Higher Chenistry Homework Unit Two - Natures Chemistry Esters, Fats and Oils 1. Which of the following structural formulae represents a primary alcohol? 2. Aspirin is one of the most widely used pain relievers in the world. It has the structure: Which two functional groups are present in an aspirin molecule? A B C D Aldehyde and Ketone Carboxyl and Ester Ester and Aldehyde Hydroxyl and Carbonyl 3. An ester has the following structural formula; CH3CH2CH2COOCH2CH3 The name of this ester is A B C D propyl propanoate ethyl butanoate butyl ethanoate ethyl propanoate 4. Rum flavour is based on the compound with the formula shown It can be made from A B C D ethanol and butanoic acid propanol and ethanoic acid butanol and methanoic acid propanol and propanoic acid 5. Which of the following compounds is hydrolysed when warmed with sodium hydroxide solution? 6. Two flasks, A and B, were placed in a water bath at 40 ˚C After several days the content of both flasks were analysed. Which results would be expected? A B C D Flask A contains ethyl ethanoate, water, ethanol and ethanoic acid; Flask B is unchanged. Flask A contains only ethyl ethanoate and water; Flask b is unchanged. Flask A contains only ethyl ethanoate and water; Flask B contains ethyl ethanoate, water, ethanol and ethanoic acid. Flask A and Flask B contains ethyl ethanoate, water, ethanol and ethanoic acid. 7. Which of the following is most likely to be used as flavourings? A B C D CH3CH2CHO CH3CH2CH2COOH CH3CH(OH)CH2CH3 CH3CH2CH2COOCH2CH3 8. Which of the following represents the structural formula for glycerol? 9. Fats have higher melting points than oils because comparing fats to oils; A B C D Fats have more hydrogen bonds Fat molecules are more saturated Fat molecules are more loosely packed Fats have more cross-links between their molecules. 10. In the formation of ‘hardened’ fats from vegetable oils, the hydrogen A B C D causes cross-linking between chains caused hydrolysis to occur increases the carbon chain length reduces the number of carbon – carbon double bonds 11. One of the chemicals released in a bee sting is an ester that has the structure shown. This ester can be produced by the reaction of an alcohol with an alkanoic acid. (a) Name this acid. (1) (b) The ester can be prepared in the lab by heating a mixture of the reactants with a catalyst. (i) Name the catalyst used in the reaction. (1) (ii) Explain why a water bath is used to heat the reaction mixture? (1) (iii) What improvement could be made to the experimental set-up shown in the diagram? (1) (iv) State two pieces of evidence that indicate that an ester has been formed. (2) (6) 12. A compound in the headache tablet aspirin has the following structure; (a) What is meant by hydrolysis? (1) Headache tablets which are kept for many months, especially in hot and humid climates, often smell of vinegar (ethanoic acid). (b) Draw the two products of the hydrolysis of aspirin. (2) (3) 13. Fats and oils are ester molecules known as triglycerides. The structure of a fat molecule is shown below (a) When this triglyceride is hydrolysed, a fatty acid is obtained. Name the other product obtained in this reaction. (1) (b) Oils are liquid at room temperature; fats are solids. Why do oils have lower melting points than fats. (1) A fatty acid is a long chained carboxylic acid. Examples of fatty acids are shown in the table below (c) Describe a test, with expected results, that could be used to distinguish between stearic acid and oleic acid. 2 (4) 14. A team of chemists are developing a fragrance for use in shower gel for men. (a) To give the gel a fruity smell the chemists are considering adding an ester. They have synthesised six isomeric esters. Volunteers smell each ester and give it a rating out of one hundred depending on how fruity the smell is. (i) Name the ester with the fruit-smelling rating of 92. 1 (ii) Shown below are the structures of three more isomers. Arrange these esters in order of decreasing fruit-smell rating. 1 (2) 15. The structure of a molecule found in olive oil can be represented as shown. (a) To which family of organic compounds do triglycerides belong? (1) (b) Olive oil can be hardened for use in margarines. What happens to the triglyceride molecules during the hardening of olive oil? (1) (2) 16. Benzoic acid, C6H5COOH, is an important feedstock in the manufacture of chemicals used in the food industry. (a) The ester ethyl benzoate is used as food flavouring. Ethyl benzoate can be prepared in the laboratory by an esterification reaction. A mixture of ethanol and benzoic acid is heated, with a few drops of concentrated sulphuric acid added to catalyse the reaction. (i) Suggest why the damp towel is attached to the opening of the test tube. (1) (ii) During esterification the reactant molecules join by eliminating a small molecule. What name is given to this type of reaction? (1) (iii) Draw a structural formula for ethyl benzoate. (1) Total 30 marks (3)