Ethers and Thioethers
advertisement

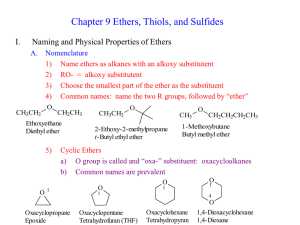
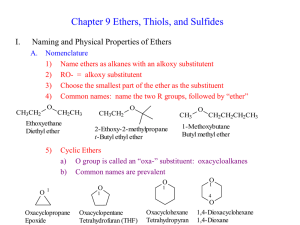
Ethers and Thioethers Bond angles at oxygen are sensitive to steric effects O O H H 105° 108.5° O O CH3 CH3 112° H CH3 C(CH3)3 (CH3)3C 132° Functional Class IUPAC Names of Ethers name the groups attached to oxygen in alphabetical order as separate words; "ether" is last word CH3OCH2 CH3 ethyl methyl ether CH3CH2OCH2CH2CH2Cl 3-chloropropyl ethyl ether CH3CH2OCH2 CH3 diethyl ether Substitutive IUPAC Names of Ethers name as alkoxy derivatives of alkanes CH3OCH2 CH3 methoxyethane CH3CH2OCH2CH2CH2Cl 1-chloro-3-ethoxypropane CH3CH2OCH2 CH3 ethoxyethane An oxygen atom affects geometry in much the same way as a CH2 group most stable conformation of diethyl ether resembles pentane An oxygen atom affects geometry in much the same way as a CH2 group most stable conformation of tetrahydropyran resembles cyclohexane PREPARATION CH3CH2CH2CH2ONa + CH3CH2I CH3CH2CH2CH2OCH2CH3 + NaI (71%) Ethers resemble alkanes more than alcohols with respect to boiling point boiling point 36°C 35°C O OH 117°C Intermolecular hydrogen bonding possible in alcohols; not possible in alkanes or ethers. Ethers resemble alcohols more than alkanes with respect to solubility in water solubility in water (g/100 mL) very small 7.5 O OH 9 Hydrogen bonding to water possible for ethers and alcohols; not possible for alkanes. Summary of reactions of ethers . Ethers are relatively unreactive. Their low level of reactivity is one reason why ethers are often used as solvents in chemical reactions. Ethers oxidize in air to form explosive hydroperoxides and peroxides. REACTIONS CH3CHCH2CH3 HBr OCH3 heat CH3CHCH2CH3 + CH3Br Br H (81%) H H CH3OCH3 H+ CH3O+CH3 H Substitutive IUPAC Names of Sulfides name as alkylthio derivatives of alkanes CH3SCH2 CH3 methylthioethane SCH3 (methylthio)cyclopentane CH3CH2SCH2 CH3 ethylthioethane Functional Class IUPAC Names of Sulfides analogous to ethers, but replace “ether” as last word in the name by “sulfide.” CH3SCH2 CH3 ethyl methyl sulfide CH3CH2SCH2 CH3 diethyl sulfide SCH3 cyclopentyl methyl sulfide Preparation of RSR' prepared by nucleophilic substitution (SN2) – S •• •• R •• CH3CHCH + CH2 R' X NaSCH3 R •• S •• R' CH3CHCH methanol Cl SCH3 CH2 R •• S •• R' Oxidation of RSR' •• – •• O •• + R S R' •• •• – •• O •• R ++ S R' • O •• • •• – sulfide sulfoxide sulfone either the sulfoxide or the sulfone can be isolated depending on the oxidizing agent and reaction conditions Sulfides can act as nucleophiles R •• S •• R' + R" X R + •• S R' product is a sulfonium salt R" X–