Ch. 1 Organic Compounds PP
advertisement

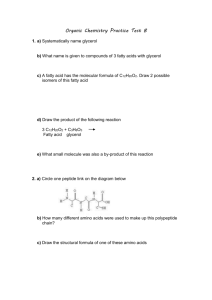
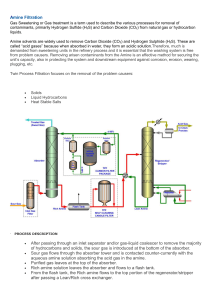
The First 10 Alkanes Alkane bond angles b/w carbons, 109.5° Structural Formula, Octane Different Diagrams/Forms for drawing Hydrocarbons Structural formula Cycloalkane Structural Isomers butane methylpropane Naming Alkanes Boiling/Melting Point Increases as Alkane size increases Uses of Alkanes Unsaturated Hydrocarbons Rules for Naming Alkenes/Alkynes Stereoisomers Cis and Trans Isomers Hydrogenation Addition Reactions Addition reaction with halogen Halogenation Hydrohalogenation Reaction Hydration Reaction Markovnikov’s Rule 2 – bromobutane is the most likely product Markovnikov’s Rule Michael Faraday (1791 – 1867) Benzene Common representation of Benzene 1-ethyl-4-methylbenzene Toluene, methylbenzene Substitution reactions involving Benzene Ethanol Primary Alcohol (1°) Secondary (2°) Alcohol Tertiary (3°) Alcohol Naming/Drawing Alcohols Hydration of Ethene to form Ethanol Dehydration Reaction of Propanol Typical Ethers Condensation Rx to form an Ether Rules for Naming Ethers - IUPAC Ethanethiol Carbonyl Group Aldehyde Aldehyde - butanal Ketone Ketone - Propanone Oxidation of a Primary Alcohol produces an Aldehyde Oxidation of a Secondary Alcohol produces an Ketone Hydrogenation Rx of Aldehydes and Ketones Carboxyl group Citric Acid Methanoic Acid (or Formic Acid) Ethanoic Acid (or Acetic Acid – Vinegar) Propanoic Acid Benzoic Acid Melting Points of Carboxylic Acids vs. Alkanes Carboxylic Acid Formation Ester Esters Ester General Esterification Esterification Glycerol Saturated and Unsaturated Fatty Acids Structure of a Fatty Acid Triglyceride formation Triglyceride Cis vs. Trans Fatty Acids Amine Primary Amine - Ethanamine Aniline Secondary Amine – N-methylmethanamine Tertiary Amine – N,N-dimethylmethanamine Boiling point of 1°, 2 °, 3 ° amines 1° Amine Synthesis 2° amine synthesis 3° amine synthesis Amide Primary Amide Synthesis Synthesis of Amides








![by AND ITS USE AS A REAGENT POR in partial iu]iiUmeut o](http://s2.studylib.net/store/data/012036276_1-9871e7b037278074a82c8ce71a7d76e4-300x300.png)
