by AND ITS USE AS A REAGENT POR in partial iu]iiUmeut o
advertisement
![by AND ITS USE AS A REAGENT POR in partial iu]iiUmeut o](http://s2.studylib.net/store/data/012036276_1-9871e7b037278074a82c8ce71a7d76e4-768x994.png)
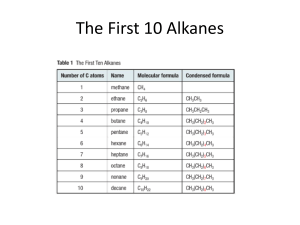
2.NITRO- 1, 3..INDNDIQNE AND ITS USE AS A REAGENT POR TRE IDENTIFICATION OF ORGANIC BASES by submitted to in partial iu]iiUmeut o the requirements or the degree oZ MASTER OP SCIENCE June 1947 Redacted for Privacy Redacted for Privacy Redacted for Privacy Redacted for Privacy cria*ne, TABLE OP CONTENTS ......,.... 1 GeneralDisoussion...... Table 1. The yield of 2-nitro-1,3-indandione secured from 2 grams of 1,3-indandione under various conditions. Rxperimental . * . . . . . * . . . . .4 . , . . . . . . . . . . . . . . . . 6 alipbatioamines..........,,....8 Table 2. The 2-nitro-1,3-indandionates of the Table 3. The 2'nitro-1,3-indand.1onates of the aromatic amines . . . . . . . . . . . . . * . . 10 Table 4. The 2-nitro1, 3-indandionates of the heterocyolicamines. . * . ... . . . . . . .13 DiscussionofResults............... Bib1iophy . . . . * . . . . . . . a . a . . . 15 . 17 2'.NiTRO-1,3INDANDIONE AND ITS USE AS A RBAGT POR THE IDENTIFICATION OP ORGANIC A8ES The mo8t uaeful type derivative of primary and secondary axnines are the amides, phenyithioureas, and sulfon- amides; and of the tertiary amines, the picrates and the quaternary ammonia salts, Bensene sultonyl chloride is probably the most frequently used reagent since it is employed in both the preparation of these derivatives and in the usual procedure (Hinaberg's method) for classifying Amines. In 1936, G. Wanag (3,4) reported a new reagent, 2-nitro-1,3-indandione, which forms salts with both the inorganic and organic cations, suoh as the simple aliphatic, aromatic, and heterocyolic wnines. He found these com- pounds to be crystalline, non-hygrosoopic, shaz-melttug salts that were very soluble in water. Later invest. tigatora at Wanag' a suggestion used this strong acid to prepare certain derivatives of the alkaloids (2) and amino acids (1). The fact that many of these salts hydrolyze to such an extent in water that a neutralization eqyivslant may be determined by standard procedures was not realized by Wanag. This property, coupled with its ease of salt formation, uon-byGxosoopic character, and sharp-melting point range makes it appear to be an unusually promising reagent for the identification of orrnic bases. Although neutralization equivalents may also be oh tamed with the hyirochlorides of' the bases, these, sales are for the most part hyGroscoic and therefore totally unsuL table for equivalent eight determthatlori. The salts were orinally repsred by alcLLng an aqueous solution of the acid to a dilute hydrochloric acid solution of the base, However, as 2-nitro-1,3 indandione itself' is very irsoluble in dilute hyrochloric acid, imoure salts were obLaied that hau to be recrystallized sev;ral times. Since the precipitation proce- dures were not unif'ori, and orouucts in'pure, a study of the salt fornaatiori in vsrious solvents was undertaken. In the course of this work it was discovered that very pure salts of 2-nitro-1,indandione could be orecipitatod from acetone solution of the base or its hydrochloride. 2-Nitro-i,3-indandione was first nrepared () by the direct nitration of l,-indandione with cold fuming nitric acid in glacial actic acid. hanag, reortiog yields of' 78, found that the conditions for this re- action vero very c itical since the temperature at which nitration takes the same. lace and oxidation occurs is nearly The critical conditions were eonfired in this labor:tory; but the yields were uncertain and were never greater than 64. i3eeause of the uncertanties of this reaction :anag also attempted to prepare it by oxidizing 2-nitroso-1, 3-indanuione with concentrated nitric acid and by condensing sodium nitrite with 1, 3-inuandione at 800 - 900 C. (6) to give tho sodium salt of 2-niro- l,5-indandione. In ecch istsnce the yields re low. In view of the poor or erratic results using fuming nitric ada and the promise of 2-nitro-1,3indandione as an organic reagent a further seudy of the direct nitration of l,--indandiono was made. Concentrated nitric acid was used as the nitrating ageat in place of fuming nitric acid since initial work indicated a greater difference between the nitration and oxidation temperatures. Table 1 summarized the results obtained upon the nitration of two grams of 1,3-Indandiane under various conditions. Incomolete nitration takes olace if the mixture is not heated to at least 450 C., or if the nitric acid concentration Is diminished. If the mix- ture Is heated over 50 C. but under 730 C. a large amount of a yellow by-product ;ieIting at j350 c results. Complete oxiu.atioii to pLthalIc acid occurs i1 the rLitration is allowed to roceed at emeratures above 80° C. Partial oxidation also occurs if the nitric acid content is increased. The light yellow precipitate of 2-nitro-1,3-indandione hydrate must be filtered rapidly and ithout agitation, as proLonged contact with the 4 Table 1 The yield of 2-nitro-1,3-indandione securea from two Rrans of 1, 3-indaine under various conditions. Temperattire 30 40 48 56 60 40 40 40 40 40 40 40 40 Time cooling minutes 60 30 60 60 60 60 30 90 120 60 60 60 60 60 Acetic Nitric ml. ml, acid 20 20 20 20 20 20 20 20 20 15 10 25 30 20 yield c1d 2 2 2 2 2 2 2 2 2 2 2 2 2 3 did not nitrate 76 84 70 some oxidation 25 traces 76 78 57 75 23 79 62 38 5 nitrating iixture causes a cor Neither can he sondin, loss in yield. recipit5te be filtered too soon as uf- ficient tI.e nust be allowea for the precipitate to forr. l,3-inoandioeiray also be nItrtet with soaiu nitrate rlacial acetic acid at 1000_1050 c., or.with nitrogen tetroxide In obloroforc.; but as the yields vre poor these latber .ethods were abandoned. 2-Nitro-1,3-indanaione can be 'eadily bro'inated (s) or chlorinated. (5) to fori the 2-halo-2-nitro-1,3indandione. However, it can be neither nitrated, nitro- sted, benzoylated, or acetyla d. It decoposed up3ri oxidation or upon the action of concentrated base or acid. to fri phtbaiic acid or Since the hthalic anhyuride. ormu1a In Figure 1 cannot satisfactori.Ly explain the acidic chaxcter of 2-nitro-1,3-indandione, 0 c/sc II I I ,° OH NV Fiure 1 Finire 2 Ehioi- form Keto-- form 0 ciç °r"II I 0 I Figure 3 Keto-nitric acid form two aduitional structures have been proposed (u). dow- ever there appears to be no direct evidence to substantiate either the enul-fora (Figu'e 2) or the keo-nitric acid for.n (Figure 6). 2-NITR0l,3-IbNi.IOL.: Twenty grams of 1,3-indan- diono and 200 milliliters of r1acial acetic acid are warmed in an ir1enoeyer flask on a water bath to about 480 0, To this is added in a siie aniouiith constant aitatiof1 twenty miililters of concentrated nitric acid (st. g. 1.42). After about five seconds he reaction oixture, which darkens considerably, is cooled rapidly with. runriin wster or in an ice bath to 100 C. After one hour at t is tern erature the yellow preciitate of 2-nitro-1,3-indandiorio is removea by suction filtration and is washed ith a little cold acetic ada. The yield aftei recrystwlization from a little water is rams (77-84) of the yellow hydrate, contdnin one mole of rater ana elti at 1130 C. Upon dryinp, in a vacuum desiccator over sulfuric acid all the water of 22-24 crystallization is lost, end the 2-nitro-1,3-indanaione is transformed into an intense yellow, anhydrous oowder, whici cannot be recrystallized from an anhydrous solvent. If the nitratimp; mixture is poured into water after rernovinn the 2-nitro-1,3-indanuio;e a yellow comound that melts at C. and contains 5,19 nitrogen is secured. As the c000und could not be kept in the crysailic bate notbig fuxther as uone Wit lb. 1350 7 SALTS OF 2-NITRO-1,3-ThDANDIONE: To a solution of the organic nitrogen base or base hydrochloride in acetone is added a slight excess of a saturated solution ol 2-nitro-1,3-indandione. (The solution is pre- pared by adding five grams of the acid to 150 ml. ol' acetone. The solubility of 2-nitro-1,3-indidione in acetone is 0.025 g./ml. at 18° 0,; and 0.032 g./ml. at 24° C.) If the precipitate does not foxm immediately the solution is evaporated at room temperature to about one-half of its original volume. The salts are recrys- tallized from 95% ethyl alcohol, water, or a combination of both. The salts thus prepared are very pure and melt within a range of one degree. A list of the salts prepared with their corrected melting points, analysis, and solubility in various solvents is to be found in Tables 2, 3, and 4. The salts formed from bases which were so strong as not to permit the determination of neutralization equivalents are marked N.T. (not titratable) in the table. The values which are reported agree within 2% of the theory. Table 2 The 2-nitro-1,3-inc&aridionbs of the a11;:hz.tic amine a, the 2-nitro-1,3-inctandionate of m.p. 00. N.E. : N caic. found soub10 in - Si pie: Methyl aniine (4) thy1 amine (4) n-Propyl amine (41 io-Propy1 amine nr3utyi amine iso-Butyl amine (4) i-Amy1 amLne iso-Amyl amine isc-Hexy1 amine . 205 203 185 205 147 N T. M m J. '. N T. 1 7 158 162 155 ri-Heptyl amine (4) 150 n_Heoty1cteer1 aniae (4)110 Allyl amine (4) 181 Cyclohexyl amine (4) 213 Camphy]. amine (4) 1o9 Bornyl amine (4) 211 thylenedi amine 222 Ethy1enedia'ine (4) 205 Propyl enecii am Inc 206 Putrescine (1) Cadaverine (1) £)i-net,hr1 amine (4) 210 Di-ethyl amine (4) 1'1 Di-proy1 amine (4) 210 ui-isoproy1 amLe 237 Di-n-butyl amine 204 Di-iso-butyl amine (4) 231 Di-sec.-buty1 amine 234 Di-n-Amyl amine 137 Di-isoamyl amine (4) 190 Di-n-hexyl amine 117 Di-isohexyl amine 133 D.-cyc1ohtxy1 amine 235 Di-n-h.eotyl amine 107 Di-n-octyl aine 91 D1-F-ethy1-hexy1 amine 144 Di-n-noyi amine 83 Di-n-decyl ai.e Sb Tri-meehyl (4) 162 .Tri-t:'j1 a.ie(4) oil ri-isohuty1 avIno (4) lii j.. A.. 1. 2. N. T. 12.54 12.84 11.89 11.40 11.2 11.O 11.22 10.62 10.b2 10.07 10.07 9.59 9.17 11.30 10.64 10. 9a73 10.24 9.2, 9.28 o. .. I. 11.32 11.00 10.00 9.68 8.43 8.22 8.14 7.60 13.08 13.06 12.67 1.80 j\ 2. A-;. N T. _4 S 'K i-' £ : £ fl 1',. Le 1T '1 . a.. 1e A I. . L, 12.28 12.03 11,92 12.03 11,57 11.20 11. 11.20 10,ol 10.83 9.5? 9.85 9.59 8.75 8.75 3,75 8,05 8.05 7.45 7.45 7.45 6.92 6.50 o.50 '.09 9.61 8.79 9.10 8.81 8.00 8.05 7.40 7.32 7,48 7.01 6,61 6.44 6.11 5.81 5.73 11.20 11.40 8,91 t.-5 rj isz (d i c, : 1.j 'J 4.J * * * * * * ** * * * * * * * * ** * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * .:? * * * * * * *** Table 2 continued The 2-nitro-1, 3-in,iandionats o the au hat1 c arni ne a. the 2-nitro-1,3-inthm- m.t. OÔ N.. dionate of N caic. found soluble in - tU S.) Alcohol: 187 Ethanol aiiine 13: Triethanol amine 2,3-Die nlno-1-pronanell95 N.T. N. 11. N. ie Hydro xye thyl ethylene- 184 dinuine 2, 3-Dihydroxy-l-oro2yl 176 amine 2, (2_aino ethoxy) ethanol 119 11.16 11.11 8.23 8.13 11.86 11.69 * * * 11.51 11.43 * * N. 2. 9.93 9.97 4' N. 1. 9.46 9.31 * Acid: Glyclne (1) b-Alanine (1) Arni de: Guanidine (4) 256 240 Urea Phenyl urea 163 140 AcetaNiladiNe (4) Arrinine (1) Wethyl uaridine (1) Inidazoyl: Histamine (1) His tidine (1) Carnosine (1) 251 32 22.41 22.30 16.87 16.84. * * * 16.73 16.24 12.84 12.87 * * * * * 10 Table 3 The 2-nitro-1,3-inua es of the ar.atio ion a 1nes. the 2-nitro-1,3-inancilonste of rn,p. "C. N.. N caic. found so1b1e in -- - -L Simple: Aniline (5) o-Toluiciine (3) m-Toluidirxe (3) 9-TOluidifle (3) 209 198 194 193 192 Xylidine 1,s,4 (4) 185 1,3,2 (4) Xy1idir 196 Xy'id1e 1,4,2 (4) 216 Xylidine 1,3,5 (4) 1 0 Benzyl azine (4) a-Phenyl ethyl a!ii1e(4)207 b-Pienl ethyl 2u .rnine(4)169 Benzedrine Benz xyiry1 amine (4) o-Ethyl aniline (4) p-thii antlise (4) o-i1LobLntny1 (4) n-Ainobiheri1 () p-AnLnohiphezyJ. (3) a-Naphthjlaine (4) b-Nao}tthy1ai:t (4) 193 205 183 i:1 163 19 190 210 193 Le r ah j-iro - a-na itthl 204 azzLne-ar () Te.tralyro-b-ria;hthyi a.tie-ac ( o-Phenr ieri a;.iie ( 4 ) 174 m-Phen-leniaAne (4) 200 p-Phenylendiine (4) 263 ) To1iyleeJiaue-1, 2,4 183 195 2, 7-iJianino flroene( 4)240 213 Benziuine (4) 216 o-T,lldine (4) 203 iibenziaiLie (4) (4) 2-Amno I'lorerie (4) N-Izobut7l benzyl amine220 N-ne ebvi aniline (0) 106 328 9.86 9.40 9.40 9.40 8.98 8.98 8.98 o.90 6.39 8,97 8.97 8.59 7.52 8,9b 8.98 7.78 7.78 7,16 8.39 8.30 9.94 9.47 9.57 9.71 o39 9.10 9,46 9.41 6.65 8.97 -.47 8.o4 7..3 9.33 9.16 7.60 8.10 7.71 .58 8.81 * * * * * * -z * * * * * * * * * 8.28 8.52 14.05 14.20 11.3 10.87 11.4 11.20 * * * 7.56 7.72 9.09 9.70 9.90 10.30 9.44 .Gf 7.22 t76 7.91 9.40 9.27 '- * * * * 8.b 8.01 11,13 11,33 QJ * * * * * * * * * - 1L Table 3 continued The 2-nitro-1,3-indandiDnates of the aromatic anies. the 2-nitro-1,3-indandionato of m.p. °C. N... N caic. found soluble in - SLp1e: N-ethyl aniline (4) N-propyl aniline (4) N-butyl anilLie (4) 8.98 8.59 8.24 8.24 18$ 191 209 N-isobutl aniline (4) 207 N-methyl-o-toluldine (4)190 N-ethyl-o-toluidine (4)192 8.8 N-iiethyl-a-naDh hyi amIne 199 N-inethyl-b-naphtthyi amine 177 amIne (4) 8,62 8.84 8.05 7.93 * * * * * * * * * * * * * * . i. ia L. c . j. S Dimethyl aniline (3) 133 o-DIiethy1 toluidine( 4)150 p-Dlcthyl toluluine (4)149 a-DIethyi naihthyl 9,0. 8.59 .59 N-ethyl-p-toluldine (4 )1ô4 8.82 8.54 8.29 1. 153 * 8.05 .32 * * :;.59 7.9]. 9.07 8.43 8.21 * * * * * * 7.73 7.65 * * * * 8.96 * Phenol: o-Ainophnol m-Aminophno1 p-Aftopheno1 ,4-Liainoinol 1-amino 2-nahthl 205 210 - 200 Ether: o-Phenotldtna m-Phenetidine p-Phenetidine o-Anisidine m-Anisiuine p-AnisLiine o-Dianisidine Halo en: o-Chloroariiline rn-Chloroaniiine p-Chioroaniiine 5-lono 2-toluluine 206 210 i20 18 205 203 226 182 192 188 lT2 300 300 300 315 350 * * * * 328 328 328 314 314 314 313 * * * * 316 * * * * * * * * 3m 31$ 424 * * * * * * * * * * 12 Table 3 continued The 2-nitro-i,3-indandionates of the aromatic amine3. the 2-nitro-1,3-indan- m.p. OQ, dionate of N.. soluable in N caic. found 1 Nitro: 162 o-Ni troaniline 132 m-Nitroaniiine 175 o-Nitroaniiine 3-Iitro-4-aminoto1uene 156 Ketone: 2-Arnicoaceterohenone 4-AirLicoaeetohenone p-Diniethyl aninobenzalaehyao 134 199 329 329 329 343 * * * * * * * * * * * * 326 323 * * 134 ** ** oil oil ,p' -Potraethy1da'Ino- benzonbeione Acid aric acid de'ivatLve: m-Aminoberizoic acid o-Ainobenzoid acid p-kminobenoic acid Ethyl anthranilate Methyl nthraniiate p-AmIno acetinil1ie(4) Benz&iciine (4) Sulfa ilaniae o-Toluidiue-5-sultorii c acid 212 159 213 197 137 212 19c 214 - 14 164 358 34. 363 395 208 Antbrani1aicte Azo and azine: 145d 295 3enzalazine 375 Tiycirazobenzene Direthji a; 1noazobenene18B Diazoa1inobenzene 191 2c;9 11.77 12.15 13.51 13.52 * 7.71 7,s4 * * 7.09 6.59 ** 7.11 .36 * * 9.49 953 11.20 11.01 * * 13.4t 13.41 12.09 12.31 * * 13 Table 4 The 2-nitro-1,3-indan<iinates of the heterocyclic amines, - the 2-nitro-1,-inda;- dionate of ni.p. 00 SiraiDle: l6 Pyridine (4) 161 a-Picolino (4) 146 b-Picoline 175 g-Picoline 155 Quinoline (4) 2-Methyl quinoline (4) 157 4-Me thyl qulno1ini 6-Methyl quinolLne 7-iUethyl quinoline 8-Methyl qulnoline (4) Acridine (4) Isoquinoline 18 161 162 160 185 18? 2Pheny1, 4, ó-aiLaethyl142 pyriiui1ine Piperidine (4) yrrolicLine iioDTho11ne 12 215 d, Acid anu aciu dexivative: 171 Nicotinic acid 198 Nicotiniide 143 Ethyl nicotine N N.E. cab, found 270 10.37 9.85 9.85 9.85 .75 8.38 8.38 6,58 8.38 8.38 7.57 284 284 334 334 334 320 575 N.$. N. :. /Q 1).60 soluble in * 9.77 9,96 9,71 8,63 * * * * * * * * 8. 46 * * * * 8.3 8.39 8.17 8.11 7,60 *** * 8.94 * * * * * * * * * * * 10.15 10.20 10.68 10.89 10.07 10.22 * * * * * * * * * * 0.75 157 313 * * * * * 16i 3 * * * * 151 444 * * * * it2 365 365 * * * * oline 159 7-Methyl-S-ni tro uin136 oline 5-Methoxy-3-nitroquin179 oline 379 Creattnne (1) Halogen: 4,7-Dic1oroquLio1ine 5-rouo--ni tro- quin- olino Nitro: 5-Nitroquinolin 8-NitroquinolirAe 5-Methyl-b-ni tro quin- - 18 32 - * 379 * * * 395 * * 14 Table 4 continued The 2-nitro-1,3-inciandionates of the heterocyclic axnines. the 2-nitro-1,3-indan- m.p. dionato of °C. Amine 3 2-Amino pyriciine (4) 2,6-Diaminopyridine 2-Amino- 4-me thyl-pyrim- idine 2, 4-Dihydroxy-6-aminopyriiidine 199 235 245 177 300 - 5-Amino uracil 2-Amino thiazol 20 4(2' ,3' -Dih7aroxToroyiamine ) -6-chioro quin- 207 azoline 4(w' -hyc1roxyhery1anine )6-chloroquinazolino 168 4(2' -hydroxyethyianiine)quinazoline 243 Alkaloid: 186 Quinine (4) Strychnin (4) 226 185 i3rucine (4) Alypine (2) Berberine (2) Brucine (2) p-Phetonine/ (2) ucaine A (2) Piprazine (2) Thoo bromine (2) Theophylline (2) %N cab, found oil 3l soluble in - 14.67 14.60 * * * 318 291 * 455 **** **** 40 ** 389 * 8,14 7.94 8.00 7.42 * 7.19 6.92 * * 15 DISCU3SIOi OF ULTS 2-Ni tro-1, 3-incLandione for..is crsta1iine, sharp melting salts in acetone solution with both the simile and substituted amines, such as the amino acids, proteins, hydroxylanthes, amides, halo-, azo-, and nitroamthes. Accurate neutralization equivalents may be determined for the salts of the veak and moderately weak bases such as aniline and quinoline but not for the weak bases as the mono-, di-, and trialkyl amines, morpholixie, and piperidine. The base may be. regenented by the addition of alkali to the salt. The solubility of the salt: of the aliphatic primary and secondary amines decreases in the polar solvents and increases in the non-polar solvents with increasing molecular eight. The melting points of the salts of the iso-amines are higher than the normal amines The salts of the aromotic arid hoterocyciic amines are even less soluble in the non-polar solvents than are the aliphatic aninos. Substitution of a nethy1, ydroxy, or amino group decreases both the melting point and the solubility. The substitution of a hal:gen, nitro, or carboxyl groups increases the solubility. The keto active compounds, hydrazine, pheny1hy drazine, hydroxylamiue, sor.icarazide, give yellow salts 16 which, on slightly warming, split out 'hater to form co1ored precipitates. Usually a iixture is formed con- sistniz of salt, -azone, and -azone salt that is iripossible to &erarte. The slts are either wilte or a ltrht yellow, with the exception of the golden-brown dimethylaminoazobenzene. 17 BIBLIOGRA.PHY rnest. tber daa 2-Nitro-indandion-(1.3) ala Basenf&llungsmittel. Zeitsohrift ftr physiologisohe 1. M11er, Chernie 269:31-.2, 1941. 2. Rosenthaler, L, Basen. 2-Nitro-1.3-indandion ala Reagens aus Soientia Pharmaceutia 9:6, 1938. 3. Wanag, Gustay. 2-Ilitro-indandion-(l.3) tnd seine Saize. Berlohte der deutsohen Chemisohen Gese11haft 69B:10661074, 1936. 4. .Wanag, Gustav und Lode, Arnold. Verwendung von 2-Nitro.- indandion-(1.3) t&r die Isolierung und Identifizierung organisoher Basen. Beriobte der deutsohen Chemisohen Geselishaft 70B:547549, 1937. 5. Wanag, Gustav und Lode, Arnold. Versuohe zur Darstellung des Ninhydrin aus 2-Nltro-indandion-(1.3). Beriohte der deutsohen Chemisohen Gesellshaft 71B:l267-1272, 1938. 6. Wanag, Gustav wid Lode, Arnold. B-Oxime des Indandion- (1.3) oder 2-Nitroso-indandion- (1.3) Beriohte der deutohen Ghernischen Gesellshaft 72B:49-50, 1939.