Proteins and Enzymes
advertisement
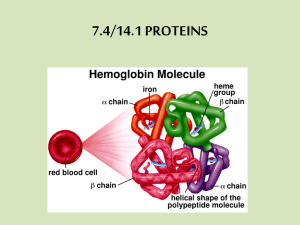
Proteins Topic 7.5 7.5 Proteins • 7.5.1 Explain the four levels of protein structure, indicating the significance of each level. • 7.5.2 Outline the difference between fibrous and globular proteins, with reference to two examples of each protein type. • 7.5.3 Explain the significance of polar and nonpolar amino acids. • 7.5.4 State four functions of proteins, giving a named example of each. Four levels of protein structure 1. Primary organization • • • • Chain of amino acids held together by polypeptide bonds 20 amino acids may be arranged in any order and is determined by DNA Primary structure determines the next three levels of protein organization (Changing one amino acid here may completely alter the structure and function of a protein, as in sickle cell disease) 2. Secondary organization • • Created by the formation of hydrogen bonds between the oxygen from the carboxyl group of one amino acid and the hydrogen from the amino group of another Most common configurations • • α-helix β-pleated sheet 3. Tertiary organization • • • Polypeptide chain bends and folds over itself because of interactions among Rgroups and the peptide backbone Important in determining the specificity of the proteins known as enzymes Interactions: • • • • Disulfide bonds Hydrogen bonds Hydrophobic/hydrophillic Ionic bonds 4. Quaternary organization • • Involves multiple polypeptide chains which combine to form a single structure Some include prosthetic or non-polypeptide groups called conjugated proteins • Ex. Hemoglobin contains four polypeptide chains, each of which contains a non-polypeptide group called a heme (contains an iron atom that binds to oxygen) Animations • http://www.johnkyrk.com/aminoacid.html Fibrous and globular proteins • Fibrous proteins • Composed of many polypeptide chains in a long, narrow shape • Insoluble in water • Examples: • Collagen – structure of connective tissue in humans • Actin – component of human muscle • Globular proteins • More 3-D in their shape • Water soluble • Examples: • Hemoglobin – delivers oxygen to body tissues • Insulin – regulates blood glucose level in humans Polar and non-polar amino acids Refers to property of R group • Polar • Hydrophilic • Found in regions exposed to water • Membrane proteins toward interior and exterior of membrane • Create hydrophilic channels in proteins through which polar substances can move • Non-polar • Hydrophobic • Found in regions of proteins that are linked to the hydrophobic area of the cell membrane Significance of polar and non-polar amino acids • Controls position of proteins in membranes, creating hydrophilic channels through membranes • Determinacy of specificity of an enzyme • Each enzyme has a region called the active site • Only specific substrates can combine with a particular active site • Combination is possible when ‘fitting’ occurs which involves general shape and polar properties of the substrate and the amino acids exposed at the active site Function Protein Description Transport Hemoglobin Contains iron that transports oxygen from the lungs to all parts of the body in vertebrates Movement Actin and myosin Interact to bring about muscle contraction in animals Hormone Insulin Secreted by pancreas that aids in maintaining blood glucose level in vertebrates Defense Immunoglobulins Group that act as antibodies to fight bacteria and viruses