Chemistry 125: Lecture 3
Sept 3, 2010
Force Laws,
Lewis Structures,
Resonance, Double Minima,
and Earnshaw’s Theorem
For copyright
notice see final
page of this file
Does Newton’s
Chemical Force Law
Exist?
How far can you Stretch
a Chain of Atoms
before it Snaps?
Force Laws & Molecular Structure
Spring (ut tensio sic vis)
F = -k x
Direct
E = k/2 (x)2
Slope
=F
2nd
Spring
(weaker,
opposing)
x minimum
Balanced
Single Minimum
Potential Energy
sum
0
0
Electrical Charges (gravity, etc.)
2
Inverse F = k / (x)
E = -k/(2|x|)
sum
x minimum !
Balanced
rd
3 Stronger
DoubleBody
Minimum
Thus with springs you might
make a stable polyatomic
molecule from point atoms.
(but not with ions or
magnets)
However, if bonds obeyed
Hooke’s Law,
they could never break.
Morse Potential
Mathematically convenient
approximation
for realistic bond energies
(proposed 1929)
Fixed
Neighbor
Sum
Second
Fixed
Neighbor
Morse Potential
Snaps at
Inflection Point
(Change from direct to inverse force)
What ARE
bonds?
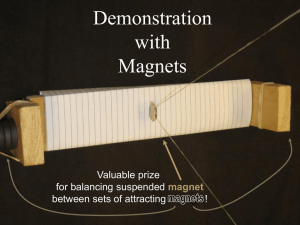
Demonstration
with
Magnets
Valuable prize
for balancing suspended magnet
between sets of attracting ma gnt!
19th Century Experiments led
to VALENCE numbers
Figure
from
1861
or 5?)
Different # for different atoms: H(1), C(4), O(2), N(3)
NH3 and NH4Cl
Why do Elements Differ?
Gertrude and Robert Robinson
(1917)“explain” so much
Such slippery concepts
convinceScheme
you of nothing.
Why/When that they
Reaction
“latent”
valence
loop ?
Why/When
“partial dissociation”?
reaction
What does the
loop mean?
How Many?
Might Latent Valence Loop explain
trivalence of pentavalent N?
Might Partial Dissociation
explain amine/HCl reactivity?
produc
t
Electron
Discovered
1897
The Cubic
Octet of G. N.
Lewis
as Harvard
as Harvard Instructor
Undergraduate
~1902
~1894
© E. S. Lewis, by permission
(1875-1946)
Octet to "Explain" Periodicity & Electron
Transfer
(1902 teaching notes)
Octet Predicts Shared Pair Bonding
shared edge
shared face
?
Cubic Octet to Tetrahedral
to Tetrahedral Octet
Octet
(G. N. Lewis 1916)
:N
N:
Tetrahedral
distribution of the
bonds from C had
already been known
in organic chemistry
for 40 years!
Good Theory should be
Realistic & as Simpleas possible
In regard to Facts
it should allow:
Prediction
Suggestion
Explanation
Classification
& Remembering
Postdiction:
Realm of Lore
From Number of Valence Electrons
we would like to predict:
Constitution (valence numbers for different atoms)
Structure (distances & angles)
Charge Distribution
Energy Content
Reactivity
Lewis Explains Constitution
“the nature and sequence of bonds”
(Electron # Valence # and Unshared Pairs)
1
H
3
•
•
B•
•
Why Octet?
Why Pair for H / He?
•
4
3
•
••
C•
•
•
N
•
••
•
HN
H
••
H
••
••
•
2
••
O
••
1
•
•
••
F••
••
HCN
•
H C N
••
• •• ••
••
HC N
•
•
•
••
H C N•
••
•
•
•
•
Bookkeeping of Lewis had the idea
+
of using : to denote
“Formal” Charges unshared pairs.
NH3 H3N-BH3 BH3
(each atom is assigned
half-interest in bonding pairs)
••
H•
•
H • • N•
•
H
H
H
+ •• ••
•• • • •
•
•
•
H N• B• H
•
•
HH
Tetravalent N
is positive.
H•
•
B• • •H
•
H
Puzzle:
Tetravalent
B
2 BH
3 B2H6 + ~40 kcal/mol
What
is the “glue”? (Answer in Lecture 16)
is negative.
+ Surface Potential* of H3N-BH
3
(from Quantum-Mechanics)
HIGH
(+ 25 kcal/mole)
N end indeed bears
positive charge
and B end bears
negative charge
(-41 kcal/mole)
LOW
*) Energy of a proton on the “molecular surface”
Lewis Explains “Pentavalent” N.
Actually Tetravalent - thus Charged.
H+
H N H Cl
H
Amine Oxide
••
••
R• + • ••
R • • N• O
• ••
R
••
••
••
••
••
one
Sulfide
oxide
R• Peroxys
++2 • • • -• •
R • • S• • O
O
•• ••
O
••
Start Lewis-Drill Problems:
Draw Lewis Dot Structures for:
HNC
(in the order shown)
also for
HCNO
(CNO in all six linear orders, plus ring)
Start Memorizing
Functional Groups
EQUILIBRIUM vs. RESONANCE
:
:
+
N closer to C
all octets
H C N O
shift
to restore N octet
shift
to eliminate charge sepn.
••
••
charge sepn
than to O
equilibrium
Geometric Implication?
all octets
• •- +
N ~midway
still charge sepn H C N O
••
between C and O
poorer site for -
••
Energy
••
but maybe
in truth…
left
N position
midway
(relative to C O)
Double
Minimum
EQUILIBRIUM vs. RESONANCE
+
H C N O
••
••
••
i.e. Notation
too simplistic
resonance
+
C N
O
••
••
H
-
••
single
compromise
position for N
Energy
Single
Minimum
left
N position
midway
(relative to C O)
Choice between
Resonance and Equilibrium
must be based on
experimental facts
(or a better theory)
that can distinguish single
from double minimum
Equilibrium vs. Resonance
A
B
A
B
One Real Species
Two Real Species
Two “Reasonable”
Structural Formulas
Failure of
Simplistic Notation
Compared to what? Typically Unusually Stable
LORE: That which
learned;
Equilibrium
vs. isResonance
learning, scholarship, erudition.
Also, in•recent
use, applied to the body
of
•
••
•
•
traditionalH
facts, anecdotes, or beliefs••
relating to some particular
H subject
C
O
O
C
O
••
•
•
• ••
•
••
O
H C
C
H
•••
•
O
O
••
••
(Oxford English Dictionary)
O
Two Species
• •
• •
LORE
O
O
H
One Species?
Species!
Two
••
• •
• •
••••
•••
•
H
H C
C
One Nuclear Geometry!
O
(Evidence:
Infrared
Spectroscopy)
(Evidence:
Electron
Paramagnetic
Resonance)
H
From a
good Text
“empirical rules for assessing the relative
importance of the resonance structures of
molecules and ions.
1. Resonance structures involve no change in the positions
of nuclei; only electron distribution is involved.
^
(our depiction of)
2. Structures in which all first-row atoms have filled octets
are generally important; however, resulting formal charges
and electronegativity differences can make appropriate
nonoctet structures comparably important.
LORE
3. The more important structures are those involving a minimum
of charge separation, particularly among atoms of comparable
electronegativity. Structures with negative charges assigned to
electronegative atoms may also be important.”
From Number of Valence Electrons
we would like to predict:
Constitution (valence numbers for different atoms)
Reactivity
Charge Distribution
O
O
••
••
O•
•
Double
Bond
O
•• • •
••O
O
••
••
Equilateral
Triangle
O
O O
O
• •
O
O•
O
•
•
O3
•
O2
Open
+
O
O
O
Trivalent O is positive.
What is Ozone’s Structure?
Ring
Open
+
O
O
_
O
O
O
A Problem in
4 Dimensions!
(3 distances + energy)
O
symmetrical single minimum?
+
O
O
_
O
Graph Help
USGS
https://webspace.yale.edu/chem125/125/xray/DensityMaps/3din2d.htm
Be sure you can do the problems,
but you don't have to hand them in.
(Click for an answer key)
Constrained
by assuming symmetry
Requires
3 4-Dimensional
/
StructureRing
Energy
Plot
e.g.
R12, R23,
Energy
R12 = R23
•
•
•
R
Energies from quantum calculations of
Ivanic, Atchity, Ruedenberg 1997
Pass
Between
Valleys
•
Open
Energy (kcal/mol)
More Constrained
2 4-Dimensional
/
StructureEnergy
Plot
O3
Pass
Ring
8
R12 ≠ R23
gives higher E
symmetrical
"resonant”
structure
Open
0
Distance along Steepest-Descent Curve
Ozone
+ in middle
- on ends?
Open
+
_
What of the charge
distribution that
is “predicted” by
Lewis bookkeeping?
O
O
O
symmetrical single minimum?
+
O
O
_
O
Suface Potential* of Open Ozone
(from Quantum-Mechanics)
+ in middle
- on ends?
HIGH
(+ 25 kcal/mole)
YES!
(-16 kcal/mole)
LOW
*) Energy of a proton on the “molecular surface”
From Number of Valence Electrons
we would like to predict:
Constitution (valence numbers for different atoms)
Reactivity (at least for H3N: BH3)
Charge Distribution (at least qualitatively for O3, H3N-BH3)
~
Structure (distances & angles) (we’ll test this later)
~
Energy Content (we’ll test this later)
Lewis Dot Structure
Attempts to provide a “physical”
basis for valence rules.
New: Reactivity from unshared pairs
(both “hooks” from the same atom)
Convenient for electron bookkeeping
(molecular charge; “formal” atomic charges;
qualitatively realistic, at least in the case of O3)
Stability and “Resonance”?
What’s so great about octets?
How bad are sestets?
How bad are structures with
formal charge separation?
How bad is “bad” charge separation?
from 2007 Wiki: “I have a question when drawing
these structures. Is it more ‘important’ to try
to fill the octet or to have lowest formal charge
on as many atoms, especially C, as possible?
and WHY?”
Is it at all True?
Are there e-pairs
between nuclei and
unshared on some atoms?
Force Laws?
by permission Sheffield University
Earnshaw's Theorem
(1839)
In systems governed by
inverse-square force laws
there can be no local minimum
(or maximum)
of potential energy.
Samuel Earnshaw (1805-1888)
Visualizing Earnshaw - Coulomb's Electrostatics
Electrostatic
Magnetic “Lines of Force”
young
Michael Faraday
by permission Alfred Bader Collection
Faraday/Davy/Phillips
Can show magnitude (as well as direction) of Force
2-D (Flatland)
Circumference
r2
force magnitude
line density
Force
line density
1/r
Can show magnitude (as well as direction) of Force
3-Dimensions
Surface
r2
force magnitude
line density
Force
line density
1/r2
In 3D such Diagrams Work only for Inverse Square Forces!
A positive particle has a local maximum or
A
minimumof
ofenergy
energyonly
(peakator
only
minimum
thevalley)
location
of
at the location
anothernever
charged
particle,
another
charged of
particle,
in free
space.
never in free space.
Earnshaw's Theorem
In systems governed by
inverse-square force laws
there can be no local minimum
(or maximum)
of potential energy in free space.
(The only “stationary” points are saddle points.)
Eppur sta fermo
“and yet it stands still”
Levitator by Martin Simon (UCLA)
End of Lecture 3
Sept 3, 2010
Copyright © J. M. McBride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting
speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0).
Use of this content constitutes your acceptance of the noted license and the terms and conditions of use.
Materials from Wikimedia Commons are denoted by the symbol
.
Third party materials may be subject to additional intellectual property notices, information, or restrictions.
The following attribution may be used when reusing material that is not identified as third-party content:
J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0