History of Quantum Theory
advertisement

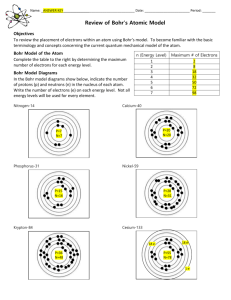
Niels Bohr Learning Goals Students will be able to: 1) understand the Quantum Mechanical Model of the Atom 2) understand how to describe the atom in terms of the Quantum Mechanical Model (energy level, shapes of orbits, sub-orbitals and quantum numbers) Success Criteria Students will: 1) record the important facts in an information chart. 2) understand the advancements of each new atomic model. 3) identify the weakness of the model that lead to further investigation. Review – Rutherford’s Model Rutherford used the Gold Foil Experiment to theorize that: 1) atoms contain a tiny, dense, positively charged nucleus. 2) the nucleus was orbited by very light, negatively charged electrons. 3) most of the volume of an atom was empty space. Limitations of Rutherford’s Model An electron accelerating around the nucleus would continuously emit electromagnetic radiation and lose energy Therefore, it would eventually fall into the nucleus and the atom would collapse However, this is not consistent with real-world observations – atoms are stable Bohr and Quantum Theory Watch Structure of the Atom 4: The Bohr Model (9:08) http://www.youtube.com/watch?v=hpKhjKrBn9s Bohr used recent work by Max Planck. Planck and his teacher, Kirchhoff, studied the light emitted from hot, dark objects (blackbodies). Planck noticed that when radiation (light, UV, IR) is emitted from a heated solid, the energy (blackbody radiation) is not released at all wavelengths, but is released at only specific wavelengths of energy Energy is quantized. It comes in chunks. Bohr and Spectroscopy A quanta is the amount of energy needed to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. Bohr looked at the spectra released by light produced by an excited gas. Bohr chose Hydrogen because it is the simplest element. https://www.youtube.com/watch?v=LA9juHlyhKw (Mr. Causey – Bohr’s Planetary Model) http://www.youtube.com/watch?v=Nv1_YB1IedE (Quantum Mechanics – The Fabric of the Cosmos – Brian Greene) A short Review on Spectroscopy – remember grade 10! (neither do I) When white light is shone through a prism, it is broken into a spectrum. Each colour corresponds to a different wavelength of light. Each wavelength corresponds to a particular amount of energy. Comparing Spectra Absorption spectra are produced when light is shone through a cooler gas. Emission spectra are produced when light is emitted by the gas. The Atomic Spectra of Hydrogen Bohr specifically used the spectra of hydrogen since it is the simplest of atoms. Spectra of Other Atoms and Luminous Objects Note that each element has its own distinctive spectra. Bohr knew that he could Bohr-ing Math measure the wavelength of the spectral lines. When hydrogen is “excited” by the addition of energy (electricity is used in a gas discharge tube), an electron jumps up from a low orbit and moves into a higher orbit. Eventually this electron falls back down and releases a specific amount of energy (a quanta). When the electrons falls back down it emits energy that we can “see” as a spectral line. (note some lines appear in the infrared and ultraviolet portions of the spectrum) Bohr-ing Math Bohr postulated that an electron cannot exist between orbits – electrons can only exist in orbits and each orbit occupies a specific energy level. Each line in the spectrum is produced by the quantum of energy released when one electron falls back down to a lower orbit. Examples: 1. drop from 4th orbital to 2nd orbital (blue line) 2. drop from the 3rd orbital to 1st orbital (ultraviolet line) 3. drop from 5th orbital to 3rd orbital (infrared line) Wavelength Equation Bohr knew that the wavelength of light could be used to determine its energy level and its velocity using the equations below: Bohr eventually expanded his math to determine the distance of each orbital from the nucleus and the energy level of each permissible energy level for hydrogen. Successes and Weaknesses of Weaknesses Bohr’s Model Successes 1) 2) 3) Bohr’s mathematics explained all the observations for Hydrogen perfectly This is a major success for Quantum Mechanics – to this day it has never failed He solved Rutherford’s problem 1) 2) Bohr’s method only worked for Hydrogen Although the quantum theory of light was experimentally proven, other experiments had proven that light had also continuous wavelike properties. Einstein suggested that there were "two contradictory pictures of reality; separately neither of them fully explains the phenomena of light, but together they do". Hence light and photons display wave and particle properties deBroglie (1924) & Schrödinger (1925) Responding to the difficulties in the Bohr model, Louis deBroglie , from France, suggested that matter like light, has the properties of both particles and waves. This particle-wave duality -derived from the work of Einstein and Planck - was experimentally confirmed, for the electron, in 1927. Austrian physicist Erwin Schrodinger formed a model of a complete atom as interacting waves. The particles became like vibrations on a violin string, only they were closed in circles. His partial differential equation seemed to bear a similar relation to the mechanics of the atom as Newton's equations of motion bear to planetary astronomy. deBroglie (1924) & Schrödinger (1925) A representation of energy levels and sub-levels as waves instead of particles in circular orbits. Note the math – Schrodinger’s wave function being applied. Heisenberg (1926) German physicist Werner Heisenberg formulated his Uncertainty Principle which says that you cannot know by measurement the position and momentum of a particle simultaneously. The better you know one, the worse you know the other. Particles and fields undulate and jump between all possible values consistent with the quantum uncertainty. Atoms were now visualized as a nucleus surrounded by a cloud of electrons distributed according to a wave pattern by the Schrodinger equation. Clouds of electrons determined by Heisenberg and Schrodinger’s mathematical models and borne out by X-ray studies. Dirac (1926) Paul Dirac devised a form of quantum mechanics (developed by Schrodinger and Heisenberg), which provides the laws of motion that govern atomic particles. The electron could now be described by four wave functions, It followed from Dirac's satisfying four simultaneous equations that the electron differential equations. As before must rotate, or spin, on its the electrons still cannot be axis, and also that there must pinpointed but exist as a sort be states of negative energy. of cloud of probability outside the nucleus. Quantum Numbers Using high resolution spectra, Michelson noticed that the main lines found in spectra were often split into smaller lines. Sommerfeld (1915) was able to explain these small lines using elliptical orbits. deBroglie and schrodinger’s work clarifies this He explained that each level has sub-levels or subshells. Therefore each one of Bohr’s energy levels can be divided into smaller levels. Secondary Quantum Number BOHR – his energy levels (orbitals) were labeled the Primary Quantum Number (n) SOMMERFELD – Secondary Quantum Number (l) l = 0 to n-1, therefore if l = 3, n can equal 0, 1, 2 Therefore the 3rd energy level contains 3 sublevels. The secondary quantum number caused orbitals to take on different shapes l = 0 (speherical orbital) l = 1 (dumb-bell shaped 2-lobed orbitals) l = 2 (4-lobed orbitals) l = 3 (6 and 8-lobed orbitals) Magnetic Quantum Number Zeeman (1896) noticed that the spectral lines could also be split if placed in a magnetic field. Sommerfeld and DeBye used this information to produce another Quantum number, the Magnetic Quantum Number (ml) ml = -l to +l Therefore if l = 1; ml can be -1, 0, +1 This means that each sublevel can have further sublevels. This causes the orbitals to have different orientations in space Spin Quantum Number Work by Pauli (1925) determine that two electrons could occupy each orbital. These electrons have opposite spins given the values +1/2 and -1/2. The Spin Quantum Number (ms) ms = -l/2 and +l/2 This means that each sublevel can be occupied by two electrons! Summary This time line draws nice comparisons between the Quantum view of the Atom with the Bohr-Rutherford model and the Lewis Structures we have used in the past. Senior Physics – TVO programs Structure of the Atom 1: The Earliest Models (9:04) http://www.youtube.com/watch?v=BhWgv0STLZs Structure of the Atom 2: Smaller than the Smallest (8:47) http://www.youtube.com/watch?v=WmmglVNl9OQ Structure of the Atom 3: The Rutherford Model (9:10) http://www.youtube.com/watch?v=FfY4R5mkMY8 Structure of the Atom 4: The Bohr Model (9:08) http://www.youtube.com/watch?v=hpKhjKrBn9s Structure of the Atom 5: Spectra (9:28) http://www.youtube.com/watch?v=5z2ZfYVzefs Structure of the Atom 6: The Wave Mechanical Model (9:08) http://www.youtube.com/watch?v=IsA_oIXdF_8 These programs provide excellent review of the History of the Atomic Model and how Quantum Mechanics is important!