Review of Bohr Models - ANSWER KEY
advertisement
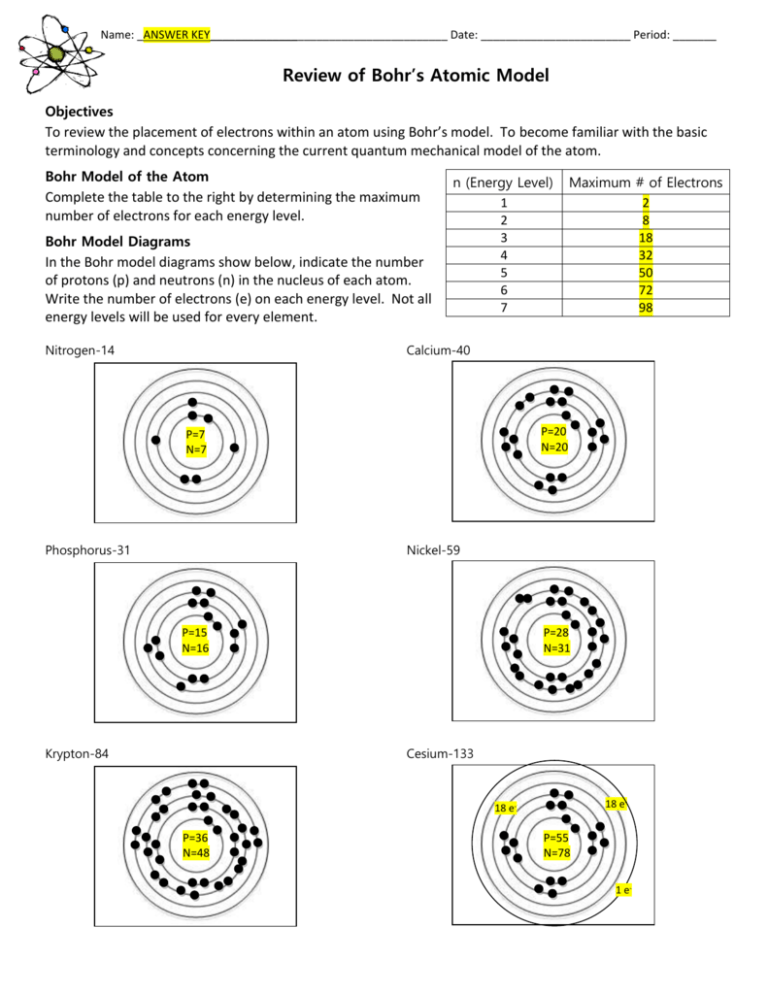
Name: _ANSWER KEY______________________________________ Date: ________________________ Period: _______ Review of Bohr’s Atomic Model Objectives To review the placement of electrons within an atom using Bohr’s model. To become familiar with the basic terminology and concepts concerning the current quantum mechanical model of the atom. Bohr Model of the Atom Complete the table to the right by determining the maximum number of electrons for each energy level. Bohr Model Diagrams In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. Nitrogen-14 n (Energy Level) 1 2 3 4 5 6 7 Maximum # of Electrons 2 8 18 32 50 72 98 Calcium-40 P=20 N=20 P=7 N=7 Phosphorus-31 Nickel-59 P=15 N=16 Krypton-84 P=28 N=31 Cesium-133 18 e- 18 e- P=36 N=48 P=55 N=78 1 e-