Class Activity
advertisement

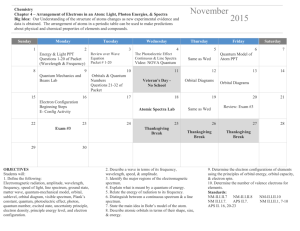
CHM 121 Class Activity – Chapter 5 Name 1. The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17 J. For light of 5.85 nm wavelength , how many photons does this energy correspond to? 2. An electron in a certain atom is in the n=5 quantum level. List the possible values of l and ml that it can have? 3. Give the orbital designations of electrons with the following quantum numbers: a. n = 3, l = 0, ml =0 b. n = 4, l = 2, ml = 0