Atom Quiz - IWBchemistry

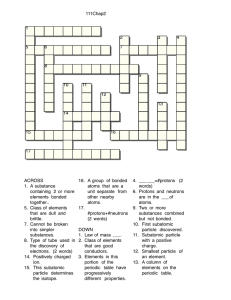
Atom Quiz
Olivia, Julia, Jaclyn, Kelly
What is a proton?
A.) a subatomic particle with no charge
B.) a subatomic particle with a negative charge
C.) A subatomic particle that has a positive charge that revolves around the nucleus
D.) A subatomic particle that has a positive charge that is located in the nucleus
What is an electron?
A.) A subatomic particle that revolves around the nucleus with a negative charge
B.) An atom of the same element that have a different number of neutrons
C.) An element that has a negative charge
D.) A subatomic particle that has no charge
What is a neutron?
A.) A element with a positive charge
B.) A subatomic particle in the nucleus with no charge
C.) A element with no charge and in the nucleus
D.) An ingredient used in Pepsi.
Where are the electrons located?
A.) Inside the nucleus.
B.) In an element.
C.) Revolving around the nucleus.
D.) In plum pudding.
How do you find the mass number?
A.) You subtract the atomic mass from the number of protons.
B.) It is the number of protons.
C.) You add the number of protons and neutrons together.
D.) You subtract neutrons from electrons
What is the atomic number for
Thulium?
A.) 65
B.) 69
C.) 176
D.) None of the above
What is one thing atoms of the same element share?
A.) The same atomic number
B.) The same shape
C.) Julia Berv
D.) The same school
What can be used to see individual atoms?
A.) binoculars
B.) 3-D glasses
C.) times 500 microscope
D.) scanning tunneling microscope
What scientist did the gold foil experiment?
A.) Darwin
B.) Rutherford
C.) Thomson
D.) Dr. Chrisophy
How are ions formed?
A.) by adding the electrons to the protons
B.) subtracting the protons from the neutrons
C.) by atoms gaining or losing electrons
D.) by combining two atoms.
Your score
10 right- you are a genius
8 right- you are extremely smart
6 right- You are doing okay
4 right – Keep trying!
2 right- You need to study more
0 right- Good Luck passing chemistry!