Chapter 12 Vocabulary words and definitions
advertisement

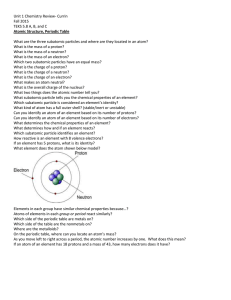
Chapter 12 Atoms and Elements Vocabulary Words and Definitions Atom - The smallest unit of an element that still has the properties of that element. Proton - A subatomic particle that has a positive electric charge. Nucleus - The center of an atom, usually made of protons and neutrons. Neutron - A subatomic particle that has the same mass as a proton but no electric charge. Electron - A subatomic particle that orbits an atom's nucleus, has a negative charge, and has very little mass. Atomic number - The number of protons in an atom. Element - A substance made up of only one kind of atom. Metal - A substance that conducts heat and electricity well and is malleable. Non-metal - A substance that does not conduct electricity and is not malleable. Periodic table - A table that shows the elements arranged by their atomic numbers. Compound - A substance made up of atoms of two or more elements that are chemically combined. Melting point - The temperature at which a substance changes from a solid to a liquid. Boiling point - The temperature at which a substance changes from a liquid to a gas. Plasma - A state of matter made up of charged atoms, uncharged atoms, and free electrons.