Activity 2 – Elements and Their Properties
advertisement

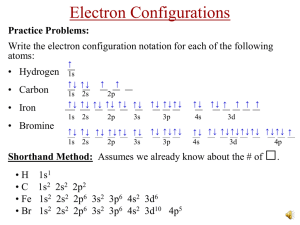
FUN WITH THE PERIODIC TABLE Conference and Interview Questions Content Activity 1 – Organizing a Store 1. What did “blank” spots represent in Mendeleev’s Periodic Table? 2. Was Mendeleev the first person to try and organize the elements? Activity 2 – Elements and Their Properties 1. Differentiate between and element and a molecule. 2. List and explain several physical properties of elements you studied in lab. 3. Compare and contrast metals and non-metals 4. Are most metals solids, liquids, or gases? 5. Discuss one of the chemical properties you studied in the lab. Activity 3 – Atoms and Their Masses 1. Compare and contrast an atom and an element. 2. Why did the single displacement reaction produce a color change? 3. Describe and explain a single displacement reaction. 4. Differentiate between a compound and an element. 5. Define and explain atomic mass Activity 4 – Are Atoms Indivisible? 1. Describe Thomson’s Cathode Ray experiment. 2. Describe Rutherford’s Gold Foil experiment. 3. Compare and contrast electrons, protons, and neutrons 4. Draw a grid representing a 250,000 chance out of one million of hitting a target. 5. Discuss, describe and draw the electron, proton, and neutron location in the atom. Activity 5 – The Electronic Behavior of Atoms 1. Why do we get different energies when the excited electrons return to different energy levels? 2. What is the fundamental difference between different colors? 3. Why couldn’t Bohr see the energy when electrons returned to energy level 1 or 3? 4. What does the “excited” state mean for an electron? 5. As energy levels move closer to the nucleus, is this a more or less stable situation? Why? 6. Describe the electromagnetic spectrum. Activity 6 – Atoms with More than One Electron 1. Explain what Ionization energy means. 2. Predict the third ionization energy for Boron. Would it be higher or lower? Why? 3. Why are Nobel Gases so stable? 4. Explain the significance by comparing and contrasting the s,p,d, and f block. Activity 7 – How Electrons Determine Chemical Behavior 1. How many energy levels are possible in atoms? 2. Why does the 4s subshell fill up prior to the 3d subshell? 3. Explain what isoelectronic means. 4. What is the relationship between valence electrons and oxidation numbers? 5. Why is Helium considered a Nobel Gas? 6. 1s2 2s2 2p6 3s2 3p6 4s2 3d7 How many valence electrons? Activity 8 – How Atoms Interact With Each Other 1. Describe and define anions and cations 2. Draw the Lewis dot structure for Lithium and Fluorine. 3. For Bromine, why is gaining one electron easier than losing 7 electrons? 4. Write formulas for Lithium Oxide, Calcium Chloride, Potassium Nitrate. 5. Name the following compounds: C3H5 MgF H2(SO4) Activity 9 – What Determines and Limits an Atom’s Mass 1. How does a nucleus stay together? Explain all forces involved. 2. Compare and contrast the Strong Nuclear Force with the Electromagnetic Force. 3. What are isotopes and give an example? 4. Why are atomic masses not whole numbers?