Metabolism of pentoses, glycogen, Fru and Gal
advertisement

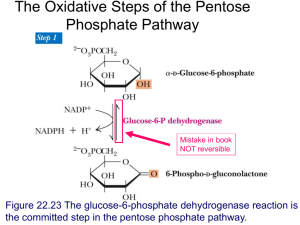
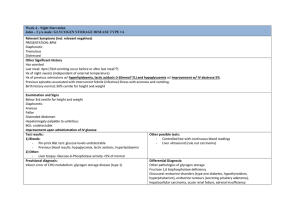
Metabolism of pentoses, glycogen, fructose and galactose Jana Novotna 1. The Pentose Phosphate Pathway The pentose phosphate pathway (PPP): (hexose monophosphate or 6-phosphogluconate patway) • Process that generates NADPH and pentoses (5-carbon sugars). • Enzymes are located in the cytosol. • Rapidly dividing cells (bone marrow, skin, intestinal mucosa, tumors) ribose 5-phosphate RNA, DNA. • Other tissues NADPH electron donor for reductive biosynthetic reactions – fatty acids synthesis (liver, adipose tissue), – cholesterol and steroid hormones synthesis (liver, adrenal glands, gonads) – elimination of oxygen radicals effects (erythrocytes). An overview: Two stages: 1) Oxidative (irreversible) • products: → ribose 5-phosphate (nucleotide synthesis) → NADPH (fatty acid synthesis, detoxification, reduction of glutathion) 2) Nonoxidative (reversible) • conversion of ribose 5-phosphate to intermediates of glycolysis • production of ribose 5-phosphate from intermediates of glycolysis 1. The oxidative phase of PPP: Regulation: Glucose 6-phosphate dehydrogenase • inhibition - by NADPH • induction - by insulin/gluckagon ↑ Some concepts • Isomers - molecules with the same molecular formula but different chemical structures (glucose and fructose) • Epimeres - differ at only one chiral center, not the anomeric carbon. • Enantiomers - chiral molecules that are mirror images of one another. Epimers Enantiomers 2. The nonoxidative phase of PPP: Pathways that require NADPH: Detoxification • reduction of oxidized glutathione • cytochrome P450 monooxygenases Reductive synthesis • fatty acid synthesis • fatty acid chain elongation • cholesterol synthesis • steroid hormon synthesis • neurotransmitter synthesis • deoxynucleotide synthesis The role of PPP in maintenance of the erythrocyte membrane integrity: Clinical correlations: Treatment by certain drugs (i.e. sulfonamides) people with glucose 6-phosphate dehydrogenase deficiency (7% of the world population) increased production of free radicals reduced protection of erythrocytes against FR hemolysis, hemoglobinuria, hemolytic anemia Summary: The pentose phosphate pathway A shunt from glycolysis Production of NADPH (reductive syntheses, detoxifications), ribose 5-phospate Conversion to intermediates of glycolysis Isomerases, epimerases, transketolases, transaldolases Glucose 6-phosphate dehydrogenase deficiency 2. Metabolism of glycogen Glycogen • The glycogen – a storage form of glucose • Required as a ready source of energy • The liver – tremendous capacity for storing glycogen – 10% of the wet weight • Muscle – max.1 – 2% of the wet weight • Muscle and liver glycogen stores serve completely different roles: – muscle glycogen – fuel reserve for ATP synthesis – liver glycogen – glucose reserve for the maintenance of blood glucose concentration Glucosyl units of α-D-glucose linked by α-1,4 and α-1,6 link (branching every 8-10 units) source of energy in animals (liver, muscles) highly branched structure (rapid degradation and synthesis, better solubility) Nonreducing end glycogenin The glycogen metabolism in the muscles and the liver: Decrease in glucose in the blood → glycogen degradation High ATP demand → release of glucose to the blood → glycogen degradation Glucose 6-phosphatase (only in liver) → anaerobic glycolysis Glycogen metabolism - an overview: Synthesis and degradation of glycogen: → different enzymes (regulation!) UDP-glucose – the substrate for glycogen synthesis and UDP is released as a reaction product glucose-1-phosphate + UTP UDP-glucose + PPi PPi + H2O 2 Pi Overall: glucose-1-phosphate + UTP UDP-glucose + 2 Pi Cleavage of PPi is the only energy cost for glycogen synthesis (one ~P bond per glucose residue). Glycogenin - (enzyme) initiates glycogen synthesis. Glycogen synthesis: A glycogen primer - 4 attached glucose molecules to glycogenin - not degraded - synthesis autocatalytic glycosylation, autophosphorylation of glycogenin) Transfer of 6-8 units Glycogen synthase (regulation) An energy-requiring pathway (UTP) Glycogen degradation: Chain cleavage (phosphorolysis) – glycogen phosphorylase - to 4 units from a branch point -The debrancher enzyme - amylo-16 glukosydase (transfer of 3 units, hydrolysis of 1 glucose) -two catalytic activities – transferase + a-16glucosydase Glycogen phosphorylase (regulation) Glycogen storage diseases: Type Enzyme affected Genetics I (Von Gierke´s Glucose 6phosphatase AR Liver (1/200 000) Hypoglycemia, lactate acidosis, hyperlipidemia, hyperuricemia. Enlarged liver and kidney. Lysosomal α-1,4glucosidase AR Organs with lysosomes Glycogen deposits in lysosomes. Hypotonia, cardiomegaly, cardiomyopathy (Infantile f.). Muscle weakness (Adult f.) The debrancher enzyme AR Liver, muscle, heart Hepatomegaly, hypoglycemia Muscle glycogen phosphorylase AR Muscle Exercise-induced muscular pain, cramps, muscle weakness disease) II (Pompe disease) III (Cori´s disease) V (McArdles disease) Organ involved Manifestations Regulation of glycogen synthase by covalent modification Regulation of glycogen phosphorylase by covalent modification Regulation of glycogen synthesis and degradation in the liver Activation of muscle glycogen phosphorylase during exercise Clinical correlations: Maternal malnutrition in the last trimester of pregnancy (physiologically: glycogen formation and storage during the last 10 weeks of pregnancy by the fetus → reserve for first hours → prevention of hypoglycemia) reduced or no glycogen reserve in the fetus after birth → hypoglycemia, apathy, coma Regulation of liver and muscle glycogen metabolism: State Regulators Response Fasting Glucagon ↑, Insulin ↓ cAMP ↑ Glycogen degradation ↑ Glycogen synthesis ↓ Carbohydrate meal Glu ↑, Glucagon ↓, Insulin ↑ cAMP ↓ Glycogen degradation ↓ Glycogen synthesis ↑ Exercise and stress Adrenalin ↑ cAMP ↑, Ca2+-calmodulin ↑ Glycogen degradation ↑ Glycogen synthesis ↓ Fasting (rest) Insulin ↓ Glycogen synthesis ↓ Glucose transport ↓ Carbohydrate meal (rest) Insulin ↑ Glycogen synthesis ↑ Glucose transport ↑ Exercise Epinephrine ↑ AMP ↑, Ca2+-calmodulin ↑, cAMP ↑ Glycogen synthesis ↓ Glycogen degradation ↑ Glycolysis ↑ Liver Muscle Summary: Glycogen metabolism Different role of glycogen stores in the liver and muscles Glycogen synthesis and degradation are separate pathways (regulation) Glycogen storage diseases 3. Fructose and Galactose metabolism Fructose metabolism Essential fructosuria Hereditary fructose intolerance Principally in the liver (small intestine, kidney) Aldolase B: low affinity for fructose 1-phosphate (→ accumulation of fructose 1-phosphate in the liver ) The polyol pathway Seminal vesicles (spermatozoa use fructose) Accumulation of sorbitol in diabetic patients Lens (diabetic cataract) Muscles, nerves (periferal neuropathy) Galactose metabolism: Lens metabolism: Diabetic cataract : ↑glucose concentration in the lens → ↑aldose reductase activity → sorbitol accumulation → ↑osmolarity, structural changes of proteins Clinical correlations: A newborn: failure to thrive, vomiting and diarrhea after milk galactosemia (Galactose 1-phosphate uridylyltransferase deficiency) genetic disease (AR, 1/60 000) hepatomegaly, jaundice, cataracts, mental retargation, death Management: early diagnose, elimination of galactose from the diet (artificial milk from soybean hydrolysate) Summary: Fructose and Galactose metabolism Conversion to intermediates of glycolysis Genetic abnormalities, accumulation of intermediates, tissue damage Accumulation of sorbitol in diabetes Pictures used in the presentation: Marks´ Basic Medical Biochemistry A Clinical Approach, third edition, 2009 (M. Lieberman, A.D. Marks) Textbook of Biochemistry with Clinical Correlations, sixth edition, 2006 (T.M. Devlin)