Cell injury vivas

CELLULAR INJURY VIVAS
2011-1, 2007-1
What is atrophy?
-
Shrinkage in the size of an organ or tissue due to decrease in cell size and number
-
Can be physiologic or pathologic, localized or generalized
-
Cells decrease in size (reduction in organelle size and metabolic needs ?permission of survival)
-
Early on have diminished function but are not dead
-
May die often by apoptosis
What are the causes and give some examples of atrophy?
-
Disuse: fracture disuse, bed rest
-
Denervation: damage to nerves causing muscle atrophy
-
Diminished blood supply: senile atrophy of the brain (athersclerosis)
-
Inadequate nutrition: cachexia
-
Loss of endocrine stimulation: post menopuase breast/reproductive organs from oestrogen lack
-
Pressure: local pressure from tumours (may be ischaemic cause also)
2010-2, 2010-1, 2007-1
What is tissue hypertrophy?
-
Increase in cellular size, but not the number of cells leading to overall organ/tissue size increase
-
Cell size increased by more structural components and increased synthesis of cellular proteins
-
Triggered by increased functional demand or stimulation by hormones or growth factors
-
Can be selective hypertrophy of specific sub-organelles
What are examples of hypertrophy (Prompt: How is it classified?)
1.
Physiological skeletal muscle enhancement through training or uterus under influence of hormones such as oestrogen, breasts in lactation
2.
Pathological such as cardiomegaly in hypertension and CCF (has an upper limit after which regression occurs -> cell injury -> apoptosis/necrosis), benign prostatic hypertrophy also
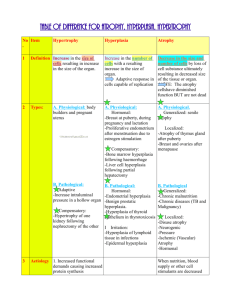
How is hyperplasia different from hypertrophy?
-
Hyperplasia involves an increase in the number of cells.
2008-1, 2007-1
What are the differences between hyperplasia and hypertrophy?
Hyperplasia: increase in number of cells in organ/tissues ually resulting in increase in volume - occurs if cellular population capable of synthesizing DNA thus permitting mitotic division
Hypertrophy increase in size of cells (structural components) causes increase in size of organs
Hypertrophy and hyperplasia often co-exis
Describe the different types of hyperplasia and give an example of each.
Physiologic: Hormonal - female breast at puberty or lactation or Compensatory - liver after partial hepatectomy, blood vessels and connective tissue for wound repair
Pathological hormonal stimulation excessive e.g. endometrial hyperplasia, benign prostatic hypertrophy caused by androgens
Note: pathologic hyperplasia is distinct from cancer b/c controlled as there are no mutations in the genes that regulate cell division, however it is described as “the fertile soil in which cancerous proliferation may arise”
2010-1, 2007-2, 2004-2
What is metaplasia
A reversible change in which one adult cell type (epithelial or mesenchymal) is replaced by another, i.e. a change in the phenoype
An adaptive change brought on by chronic stress such as chemical or physical irritation so that cells change to other cell types that are better able to withstand the adverse environment
Can you give examples
Most common is columnar -> squamous epithelium
trachea in smoking
salivary, pancreatic and biliary ducts by stones (secretory columnar changed to non-secreting sqaumous)
vitamin A def may cause squamous metaplasia in respiratory, or corneal, renal
vitamin A excess may stimulate osteoclast formation -> bone resorption and fractures
Metaplasia of squamous to columnar
Barretts oesophagus (in response to acid, predisposing to adenocarcinoma)
Metaplasia of one connective tissue type to another e.g. muscle -> bone or cartilage e.g myositis ossificans
What is the mechanism causing metaplasia
A reprogramming of epithelial stem cells or undifferentiated mesenchymal cells
involves signals from cytokines, growth factors, ECM components, genes, and DNA methylation
How may metaplasia progress 2010-1
The influences that brought about the metaplasia if persistent may initiate malignant transformation, e.g. squamous cell carcinoma in smokers, adenocarcinoma in Barrett’s
With removal of the stimulus it may undergo reversal back to the normal tissue type
2009-2
What are the morphological and chemical changes associated with early cell injury
Initially there is a reduction in function => biochemical changes (eventually irreversible injury) => structural changes (from ultrastructure => light microscopy => gross morphologic)
1.
Decreased generation of ATP
2.
Loss of cell membrane integrity
3.
Defects in protein synthesis
4.
Cytoskeletal damage
5.
DNA damage
What are the phenomena that characterize irreversible cell injury
The first is the inability to reverse mitochondrial dysfunction (lack of oxidative phosphorylation and ATP generation) even after resolution of the original injury
The second is the development of profound disturbances in membrane function
Can you give an example of a protein the leaks across degraded cell membranes
1.
Cardiac muscle - contains a specific isoform of the enzyme creatine kinase and of the contractile protein troponin
2.
Liver (and specifically bile duct epithelium) - contains a temperature-resistent isoform of the enzyme alkaline phosphatase
3.
Hepatocytes - contain transaminases
2011-1, 2009-1, 2008-2, 2006-1, 2005-2, 2004-2, 2003-2
What are the stages of ischaemic cell injury
Initial reversible
Irreversible w/ prolonged ischaemia injury and necrosis
2004-2
Describe the biochemical features of (ischaemic) cell injury.
1.
Depletion of ATP :
Sodium pump reduction w/ Na into cells , K+ out
Inc. catabolites in cells
Inc. osmotic load => swelling
2.
Defects of membrane permeability -
Leakage of intracellular substances: myoglobin, CK, troponin, other enzymes
3.
intracellular Ca2+
Initially release from intracellular stores then influx of Ca2+ across plasma
4.
Anaerobic metabolism
Lactic acid w/
pH
Free oxygen radical formation
5.
Mitochondrial damage
Decreased protein synthesis
-
Lipid breakdown products
-
Intracellular glycine
2008-2, 2004-2, 2003-2
Describe the morphological changes seen in cells with reversible ischaemia.
Reversible changes
1.
Cellular Swelling : failure to maintain ionic and fluid haemostasis; organs become swollen, plasma membrane blebs intramembranous aggregations
2.
Mitochondrial swelling, small densities
3.
Distended segments of ER with dispersion of ribosomes ‘vacuolar degeneration’
4.
Clumping of nuclear chromatin.
5.
Fatty change: lipid vacuoles in cytoplasm.
Irreversible changes
1.
Cell membrane defects
2.
Myelin figures in cytoplasm
3.
Rupture of lysosymes and autodigestion
4.
Mitochondrial large densities
5.
Lysis of ER
6.
Nuclear pyknosis, karyolysis or karyorrhexis.
2008-2, 2005-2
What is the difference between ischaemic and hypoxic injury?
-
Ischaemic involves disruption or reduction in blood supply resulting in reduced oxygen delivery, reduced delivery of substrate and reduced removal of metabolic products
-
Hypoxic involves reduced oxygen delivery only, so anaerobic (glycolytic metabolism can continue as new substrate is being delivered and metabolic wastes removed
-
As a result cellular, hence tissue injury is much more rapid in ischaemic injury
2011-1
Describe the cellular changes in necrosis
Irreversible cellular injury
Often adjacent inflammation
Swollen cells
Increased eosinophilia
Myelin figures (whorls of cell membrane bits)
Nucleus fades (karyolysis), may shrink (pyknosis) and then fragments (karyorrhexis)
Organelle disruption w/ amorphous mass
Lysosomal rupture w/ auto digestion
Cell membrane disrupted , contents released, further tissue damage and inflammation
What are the patterns of tissue necrosis?
1.
Coagulative (architecture preserved)
– the most common form
2.
Liquefactive (digestion => liquid viscous mass) – seen in the brain
3.
Caseous (friable white) – seen in TB
4.
Gangrenous - usually applied to limb, typically coagulative but may have superimposed liquefaction fro m infection ‘wet gangrene’)
5.
Fat necrosis (focal areas of fat destruction)
6.
Fibrinoid (microscopic feature of Ag-Ab complexes in vessel walls, immune mediated)
2010-1, 2008-1
What is apoptosis
Pathway of programmed cell death
Induced by tightly regulated intracellular programme
Cells that are destined to die activate enzymes that degrade the cells’ own nuclear DNA and nuclear/cytoplasmic proteins
-
The cell’s plasma membrane remains intact
Cell shrinks
Chromatin condensation
Formation of cytoplasmic blebs and apoptotic bodies
Apoptotic cell or cell bodies becomes target for phagocytosis (macrophages)
Dead cell rapidly cleared before contents leak out so this does not elicit an inflammatory reaction in the host
List some important stimuli for apoptosis
Physiologic
Programmed destruction of cells during embryogenesis
Hormone dependent involution in adult such as endometrial breakdown
Cell deletion in proliferating cell populations e.g. intestinal crypt cells
Death of host cells that have served their purpose e.g. neutrophils in acute inflammation
Elimination of potentially harmful self reactive lymphocytes.
Cell death induced by cytotoxic T cells
Pathological
Cell death secondary to radiation injury or cytotoxins
Viral hepatitis
Pathologic atrophy after duct obstruction in pancreas, parotid or kidney
Cell death in tumours
2010-1, 2006-2, 2006-1, 2005-2
What is reperfusion injury?
-
Further injury and cell death to ischaemic tissue that occurs after restoration of blood flow.
What are the proposed mechanisms of reperfusion injury.
1.
Generation of oxygen free radicals : formed from incomplete reduction of in-coming O2 by damaged mitochondria in affected tissue and action of oxidases (generated from ischaemic cells and leucocytes)
2.
Associated inflammation : cytokines, adhesion molecules generated by hypoxic cells; they recruit neutrophils etc in re-perfused tissue; ensuing inflammation causes additional injury
3.
Activation of complement system: IgM Ab deposit in ischaemic tissue; restored blood flow brings complement proteins that bind to Ab and are activated; causing further cell injury and inflammation
4.
Mitochondrial permeability transition - via reactive O2 species - effects mitochondrial function - precludes recovery of ATP / energy supplies for the cell
2008-1, 2007-1, 2004-2
Please describe the 2 different forms of pathological calcification and give an examples.
1.
Dystrophic calcification
Normal serum calcium
In necrotic or dying tissue
Examples: Atherosclerosis; calcific aortic stenosis; tuberculous node
2.
Metastatic calcification
Abnormal (raised) calcium
Deposits in normal tissues (often associated acid environment as if favours precipitation)
Examples: nephrocalcinosis; pulmonary calcinosis; gastric mucosal
What tissues are most commonly affected by metastatic calcification? 2004-2
Gastric mucosa, Kidneys, Lungs, Systemic arteries, Pulmonary veins
Describe the different principal pathological causes of hypercalcaemia, w/ clinical examples.
1.
Increased PTH secretion + bone resorption: hyperparathyroidism
2.
Destruction of bone tissue: skeletal metastases, myeloma, Paget’s
3.
Vit-D related disorders: sarcoidosis, hypervitaminosis D
4.
Renal failure: secondary hyperparathyroidism + phosphate retention
2004-2
What is steatosis?
-
Abnormal accumulations of triglycerides within parenchymal cells
Which organs are commonly involved in steatosis?
- Liver
- Heart, muscle, kidneys
What are the causes of hepatic steatosis?
Alcohol abuse
Toxins (CCl4), protein malnutrition, diabetes mellitus, obesity, anoxia, starvation, NAFLD
In the liver it results from defects in any one of the events in the sequence from fatty acid entry to lipoprotein exit (FFA esterified to triglycerides => converted into cholesterol and phospholipids or oxidized to ketone bodies => associated with apoproteins to form lipoproteins and released into the circulation)