Tissue Lecture
advertisement

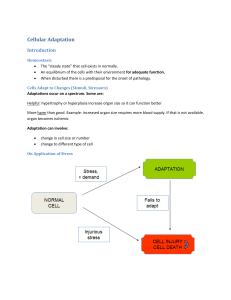
Molecular and Cellular Basis of Disease (MCBD) Cell Injury Normal cell + Stress - Stress Adapted Cell Injury Reversibly injured cell Irreversibly Injured cell Apoptosis Dead cell Necrosis Overview Normal cell + Stress - Stress Adapted Cell Cellular adaptation Normal cell +Stress - Stress Adapted Cell Stress = ? Increased/decreased workload * skeletal muscle and body building * cardiac muscle and hypertension * skeletal muscle disuse (limb immobilization) Increased/decreased stimulation * estrogenic stimulation of uterus in pregnancy * estrogen/prolactin stimulation of breast (lactation) * denervation of muscle Adapted Cell Cellular adaptations to stress 1. Hyperplasia (more cells) 2. Hypertrophy (bigger cells) 3. Atrophy (smaller cells) 4. Metaplasia (different type of cells) 1. Hyperplasia (more cells) 1. Physiologic * Hormonal (breast/uterus in pregnancy) * Compensatory (liver after partial hepatectomy) 2. Pathologic Excessive hormone/GF stimulation of target tissue * Endometrial hyperplasia (x’s estrogen) * Benign prostatic hyperplasia (x’s androgens) * Connective tissue cells in wound healing Thyroid hyperplasia Hyperplasia (Mechanism) Cell proliferation via increased production of TRANSCRIPTION FACTORS due to * Increased production of GF * Increased levels of GF receptors * Activation of intracellular signaling Results in larger organ Adapted Cell 2. Hypertrophy (larger cells) * Not due to swelling * Increased synthesis of structural components * Results in larger organ * May occur with hyperplasia Hypertrophy Comments * Often involves switch from adult to fetal/neonatal forms i.e. a-myosin heavy chain b-myosin heavy chain * Limited (can only increase so much) Hypertrophy (Heart) Hypertrophy of uterus Normal Hypertrophied Cardiac muscle hypertrophy Hypertrophy (Mechanisms) •Increased synthesis of structural proteins via •Transcription factors (i. e. c-fos and c-jun) •Growth factors (TGF-b, IGF-1, FGF) •Vasoactive agents (endothelien-1, AII) Figure 1-4 Changes in the expression of selected genes and proteins during myocardial hypertrophy. 3. Atrophy (smaller cells) 1. Physiologic During development: i.e. notochord; thyroglossal duct 2. Pathologic (local or generalized) via * disuse * Loss of endocrine stimulation * denervation * Aging * ischemia * Pressure * Nutrition Normal Atrophied Brain atrophy Atrophy (Mechanism) Reduction in structural components Decreased number of mito, myofilaments, ER via proteolysis (lysosomal proteases; ubiquitin-proteosome system) Increase in number of autophagic vacuoles Residual bodies (i.e. lipofuscin brown atrophy) NB: diminished function but not dead 4. Metaplasia **One adult cell type replaces another** Reversible Columnar to squamous epithelium (most common epithelial type of metaplasia) Chronic irritation i.e. (in trachea and bronchi of smokers) Vit A deficiency squamous metaplasia in respiratory epithelium May be some loss of function May predispose to maligancy Photomicrograph of the trachea from a smoker. Note that the columnar ciliated epithelium has been replaced by squamous epithelium. Photomicrograph of the junction of normal epithelium (1) with hyperplastic transitional epithelium (2). Metaplasia (Mechanism) Reprogramming 1. of stem cells present in normal tissues 2. of undifferentiated mesenchymal cells in connective tissue Mediated by signals from cytokines, GF or ECM Leading to induction of specific transcription factors Metaplasia versus Dysplasia 1. Dysplasia is a pathological term used to refer to an irregularity that hinders cell maturation within a particular tissue whereas Metaplasia is the process of the reversible substitution of a distinct kind of cell with another mature cell of the similar distinct kind. 2. Dysplasia is cancerous whereas Metaplasia is noncancerous. 3. Metaplasia can be stopped by removing the abnormal stimulus, but Dysplasia is a non-reversible process.