Molecular Motors
advertisement

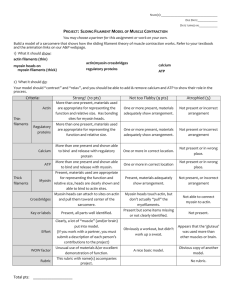
Molecular Motors Pages 1010-1034 General Characteristics of Molecular Motors Motor proteins – bind to a polarized cytoskeletal filament and use the energy derived from repeated cycles of ATP hydrolysis to more steadily along it -They differ in the type of filament they bind to, the direction in which they move along the filament, and the “cargo” they carry. Myosin II Structure Myosin was the first motor protein identified and is responsible for muscle contraction. It is formed from 2 heavy chains and 2 light chains. Myosin II Thick Filament Motor Activity is Located in the Myosin Head Myosin Superfamily Tree -Myosin tails have diversified during evolution to permit the proteins to dimerize with other subunits and to interact with different proteins or cargo. Humans have about 40 myosin genes Kinesin Kinesin is a motor protein that moves along microtubules It was first identified is the giant axon of the squid, where it functions to carry organelles away from the neuronal cell body toward the axon terminal by walking toward the + end of microtubules Many kinesins or kinesin-related proteins function in organelle movement or have specific roles in mitotic and meiotic spindle formation and chromosome separation. Kinestin and Kinestin-Related Proteins Goes towards + end Goes towards – end Increases dynamic instability Monomer Dynein Structure Dyneins are a family of – end directed microtubules motors - are the largest and fastest molecular motors Cytoplasmic dynein important for vessicle trafficking and localization of the Golgi apparatus Ciliary dynein important in the beating of cilia and flagella Myosin and Kinesin Structure -They have nearly identical cores Myosin Walking Cycle Cocked Rigor state Attached Force-Generating Released Attached Comparison of Kinesin and Myosin Motor Proteins are Adapted to Cell Functions Kinesin –moves in a highly processive fashion, traveling hundred of ATPase cycles on a microtubule before dissociating Myosin II – makes just one or a few steps along an actin filament before dissociating Two Reasons: 1. the cycles of the two motor heads in a kinesin dimer are coordinated with each other, so that one kinesin head does not let go until the other is ready to bind 2. kinesin spends a larger fraction of its ATPase cycle tightly bound to the microtubule Walking Direction of Kinesin Family Proteins The coiledcoil domain seems to determine the directionality of movement Attachment Model of Dynein to an Organelle Motor proteins also have a significant role in organelle transport along actin filaments Effect of Microtubule Depolymerization on the Golgi Apparatus Green – Golgi apparatus Red – microtubules Golgi are being positioned near the center of the cell by dyneins moving towards the – end of microtubules Myosin V on Melanosomes Black – melanosomes Green – Myosin V Melanosome Movements in Fish Pigmented Cells -Both dynein and kinesin are associated with pigment granules Regulation of Myosin II Non-muscle myosin Skeletal Muscle Cells Skeletal Muscle Myofibrils Longitudinal section The Sarcomere Sliding-Filament Model for Muscle Contration Accessory Proteins in a Sarcomere Alpha-actinin Calcium Release in the Sarcoplasmic Reticulum Regulation of Skeletal Muscle Contraction Troponin T=tropomyosin binding I=Inhibitory C=Ca++ binding Resting state I,T pulls tropomyosin out of its normal binding groove and blocks myosin Active state C binds Ca++ and releases tropomysoin allow the interaction of actin and myosin Effect of Subtle Mutations in Cardiac Myosin 6-day old mouse Flagella and Cilia Movements Microtubules in a Flagellum or Cilium Cilliary Dynein Bending of an Axoneme Structure of Basal Bodies Base of cilia and flagella, and centrioles Kartagener’s syndrome – defect in ciliary dynein