Supplementary Methods - Word file (78 KB )
advertisement

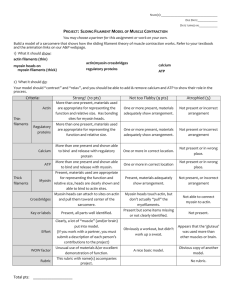
Supplementary Methods. Myosin VI expression and purification. To create a series of single-headed myosin VI constructs, porcine myosin-VI wild-type cDNA was truncated either after insert2 (Ala816; MDins2), just after the IQ motif [(Gly839; short MDins2IQ (S1)1), or (at A859; long MDins2IQ)]. For each construct, a Flag tag (encoding GDYKDDDDK) was inserted at the N-terminus (MDins2) or the C-terminus (long and short MDins2IQ) to facilitate purification. To remove insert1 for the (-insert1) construct, site-directed mutagenesis was used to delete residues (278303) in the short MDins2IQ construct. These Myosin VI constructs were expressed using the baculovirus/SF9 cell expression system. The SF9 cells were co-infected with recombinant virus expressing the myosin VI heavy chain and a recombinant virus expressing calmodulin. Details of the protein expression and purification have been published1. Kinetic studies. All reagents were the highest purity commercially available. ATP was prepared fresh from powder. N-Methylanthraniloyl (mant)-labeled ADP and ATP were prepared as described2. ATP and ADP concentrations were determined by absorbance at 259 nm using 259 of 15 400 M-1.cm-1. Nucleotides were prepared prior to use in the presence of equimolar MgCl2. Actin was purified from rabbit skeletal muscle using an acetone powder method3 and gel filtered. Pyrene labeling was done with pyrenyl iodoacetamide (Molecular Probes) as described4. All experiments with myosin VI were performed in KMg50 buffer (50 mM KCl, 1 mM EGTA, 1 mM MgCl2, 1 mM DTT, and 10 mM imidazole-HCl, pH 7) and at 25 °C. Steady-state ATP hydrolysis by MVI short MDins2IQ contructs (50-100 nM) was examined using the NADH-linked assay5,6 with a final MgATP concentration of 1 mM. Transient kinetic experiments were performed as previously described5,6, with an Applied Photophysics (Surrey, U.K.) stopped-flow having a dead-time of 1.2 ms. Pyrene actin was excited at 365 nm and mant-labeled nucleotides were excited at 295 nm, and their emission was measured using a 400 nm long pass filter. Nonlinear least-squares fitting of the data was done with software provided with the instrument or Kaleidagraph (Synergy Software, Reading, PA). Uncertainties reported are standard error of the fits unless stated otherwise. 1. Wells, A.L., Lin, A.W., Chen, L.-Q., Safer, D., Cain, S.M., Hasson, T., Carragher, B.O., Milligan, R.A. & Sweeney, H.L. Myosin VI is an actin-based motor that moves backwards. Nature 401, 505-508 (1999). 2. Hiratsuka, T. New ribose-modified fluorescent analogs of adenine and guanine nucleotides available as substrates for various enzymes. Biochem. Biophys. Acta 742, 496-508 (1983). 3. Pardee, J.D. & Spudich, J.A. Purification of muscle actin. Methods Enzymol. 85 Pt B, 164181 (1982). 4. Pollard, T.D. Polymerization of ADP-actin. J. Cell Biol. 99, 769-777 (1984). 5. De La Cruz, E.M., Ostap, E.M. & Sweeney, H.L. Kinetic mechanism and regulation of myosin VI. J. Biol. Chem. 276, 32373-32381 (2001). 6. De La Cruz, E.M., Wells, A.L., Rosenfeld, S.S., Ostap, E.M. & Sweeney, H.L. The kinetic mechanism of myosin V. Proc Natl Acad Sci USA. 96, 13726-31, (1999).